Abstract
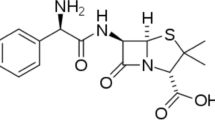
The adsorption and corrosion inhibition behavior of cefuzonam (CZM) at mild steel surface were studied gravimetrically and electrochemically by using electrochemical impedance spectroscopy and Tafel polarization techniques. The increase in concentration and immersion time showed a positive effect. Inhibitor molecules directly adsorb on the surface on the basis of donor acceptor interactions between the p-electrons of benzene, sulfur and nitrogen atoms and the vacant d-orbital of iron atoms. The adsorption of CZM followed the Langmuir adsorption isotherm. A potentiodynamic polarization study revealed that CZM acted as mixed type of inhibitor. The results obtained from different methods are in good agreement. The adsorption behavior of CZM was experimentally investigated by contact angle measurement on metal surface. The contact angle of metal surface to the acid solution increased with inhibitor concentration, thereby confirming the increased hydrophobic nature of metal surface to the acid solution having the inhibitor.





Similar content being viewed by others
References
G. Trabanelli, Corrosion 47, 410 (1991)
A.K. Singh, M.A. Quraishi, Corros. Sci. 52, 152 (2010)
A.K. Singh, M.A. Quraishi, J. Appl. Electrochem. 40, 1293 (2010)
A.K. Singh, M.A. Quraishi, Corros. Sci. 53, 1288 (2011)
A.K. Singh, E.E. Ebenso, M.A. Quraishi, Int. J. Electrochem. Sci. 6, 5676 (2011)
A.K. Singh, M.A. Quraishi, Corros. Sci. 52, 1529 (2010)
A.K. Singh, S.K. Shukla, M. Singh, M.A. Quraishi, Mater. Chem. Phys. 129, 68 (2011)
A.K. Singh, S. Khan, A. Singh, S.M. Quraishi, M.A. Quraishi, Res. Chem. Intermed. doi:10.1007/s11164-012-0677-8 (2012)
A.K. Singh, E.E. Ebenso, Res. Chem. Intermed. doi:10.1007/s11164-012-0717-4 (2012)
A.K. Singh, M.A. Quraishi, Corros. Sci. 52, 1373 (2010)
A.A. Hermas, M.S. Morad, M.H. Wahdan, J. Appl. Electrochem. 34, 95 (2004)
C. Deslouis, B. Tribollet, G. Mengoli, M.M. Musiani, J. Appl. Electrochem. 18, 374 (1988)
S.S. Abdel Rehim, H.H. Hassan, M.A. Amin, Appl. Surf. Sci. 187, 279 (2002)
A.K. Singh, E.E. Ebenso, M.A. Quraishi, Int. J. Electrochem. Sci. 7, 2320 (2012)
V.P. Singh, P. Singh, A.K. Singh, Inorg. Chim. Acta 379, 56–73 (2011)
M. Mahadavian, M.M. Attar, Corros. Sci. 48, 4152 (2006)
A.K. Singh, M.A. Quraishi, Mater. Chem. Phys. 123, 666 (2010)
A.K. Singh, M.A. Quraishi, J. Appl. Electrochem. 41, 7 (2011)
A.K. Singh, S.K. Shukla, M.A. Quraishi, E.E. Ebenso, J. Taiwan Inst. Chem. Eng. 43, 463 (2012)
A.K. Singh, M.A. Quraishi, Corros. Sci. 51, 2752 (2009)
A.K. Singh, M.A. Quraishi, Int. J. Electrochem. Sci. 7, 3222 (2012)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, A.K., Ebenso, E.E. & Quraishi, M.A. Corrosion inhibition behavior of cefuzonam at mild steel/HCl acid interface. Res Chem Intermed 39, 3033–3042 (2013). https://doi.org/10.1007/s11164-012-0815-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0815-3