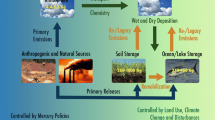
Abstract
The photolysis of nitrous acid (HONO) is an important reaction of atmospheric chemistry due to the fact that it can be the source of OH radical in the troposphere. Despite its role as a radical precursor, the chemical mechanisms leading to HONO formation are not well understood. It is commonly assumed that HONO formation is due to both homogeneous and heterogeneous processes involving NOx (mixture of NO and NO2) in which the kinetic and mechanistic details are still under investigation. In this discussion, we would like to highlight the formation of HONO from NO2 and nitric acid (HNO3) in the presence of organic particulate. We understood that in the real case, many parameters can influence the reaction mechanism; however, this is just an effort to have a better understanding of the study of HONO formation in the atmospheric process.



Similar content being viewed by others
References
M.M. Maricq, Chemical characterization of particulate emissions from diesel engines: a review. Aerosol Sci. 38, 1079–1118 (2007)
L.A. Barrie, Atmos. Environ. 20, 643–663 (1986)
A.D.A. Hansen, T. Novakov, J. Atmos. Chem. 9, 347–361 (1989)
A.D.A. Hansen, B.A. Bodhaine, E.G. Dutton, R.C. Schenell, Geophys. Res. Lett. 15, 1193–1196 (1988)
A. Indarto, Environ. Eng. Sci. 26(5), 251–257 (2009)
Z.A. Mansurov, Combust Explos. Shock Wave. 41(6), 727–744 (2005)
H. Jander, H.Gg. Wagner, Combust. Explos. Shock Wave. 42(6), 696–701 (2006)
J. Xi, B.J. Zhong, Chem. Eng. Technol. 29(6), 665–673 (2006)
M. Frenklach, J. Warnatz, Combus. Sci. Technol. 51, 265 (1987)
R.M. Santiago, A. Indarto, J. Mol. Model. 14, 1203–1208 (2008)
A. Indarto, A. Giordana, G. Ghigo, A. Maranzana, G. Tonachini, Phys. Chem. Chem. Phys. 12, 9429–9440 (2010)
A.V. Krestinin, Chem. Physic Rep. 17(8), 1441 (1998)
A.V. Krestinin, Proc. Combust. Symp. 27, 1557 (1998)
A. Indarto, A. Giordana, G. Ghigo, G. Tonachini, J. Phys. Org. Chem. 23, 400–410 (2009)
H.F. Calcote, Combust Flame 42, 215–242 (1981)
N.A. Slavinskaya, M. Braun-Unkhoff, and Frank, P, Proc. European Combust. Meet (2005)
P.A. Vlasov, and J. Warnatz, German-Russian Workshop on Reactive Flow, Berlin (2002)
G. L. Agafonov, I. Naydenova, M. Nullmeier, P. A. Vlasov, and J. Warnatz, Proc. Int. Collis Dyn Explosion Reactive Syst (2005)
J.Z. Wen, M.J. Thomson, M.F. Lightstone, S.N. Rogak, Energy Fuel. 20, 547–559 (2006)
J.Z. Wen, M.J. Thomson, and M.F. Lightstone, Proc. Join Meet. US Sect. Combust. Inst. (2005)
S. Wang, R.C. Flagan, J.H. Seinfeld, Atmos. Environ. 26a(3), 421–434 (1992)
J. Mäkela, P. Aalto, V. Jokinen, T. Pohja, A. Nissinen, S. Palmroth, T. Markkanen, K. Seitsonen, H. Lihavainen, M. Kulmala, Geophys. Res. Lett. 24, 1219–1222 (1997)
F. Karagulian, M.J. Rossi, J Phys. Chem. A 111(10), 1914–1926 (2007)
K. Lee, J. Xue, A.S. Geyh, H. Ozkaynak, B.P. Leaderer, C.J. Weschler, J.D. Spengler, Environ. Health Perspect. 110(2), 145–150 (2002)
B.J, Finlayson-Pitts, J.N. Pitts Jr., Chemistry of the upper and lower atmosphere—theory, experiments, and applications. Academic Press, San Diego (2000)
Y. Yu, B. Galle, A. Panday, E. Hodson, R. Prinn, S. Wang, Atmos. Chem. Phys. 9, 6401–6415 (2009)
F. Sakamaki, S. Hatakeyama, H. Akimoto, Int. J. Chem. Kinetic. 15, 1013–1029 (1983)
R. Svensson, E. Ljungstrom, O. Lindqvist, Atmos. Environ. 21, 1529–1539 (1987)
C.A. Longfellow, A.R. Ravishankara, D.R. Hanson, Geophys. Res. 104, 13833–13840 (1999)
A. Messere, R. Niessnera, U. Pöschl, Carbon 44(2), 307–324 (2006)
Y.F. Elshorbany et al., Atmos. Chem. Phys. Discuss. 8, S11192–S11200 (2009)
A. Rondon, E. Sanhueza, Tellus B 41, 474–477 (1988)
L.D. Ziemba, J.E. Dibb, R.J. Griffin, C.H. Anderson, S.I. Whitlow, B.L. Lefer, B. Rappenglück, J. Flynn, Atmos. Environ. 44, 4081–4089 (2010)
T.G. Koch, J.R.P. Sodeau, J Phys. Chem. 99(27), 10824–10829 (1995)
A. Febo, C. Perrino, I. Allegrini, Atmos. Environ. 30, 3599–3609 (1996)
R. Asatryan, J.W. Bozzelli, J.M. Simmie, Int J. Chem. Kinet. 39(7), 378–398 (2006)
M.T. Nguyen, R. Sumathi, D. Sengupta, J. Peeters, Chem. Phys. 230, 1–11 (1998)
G. Lammel, J.N. Cape, Chem. Soc. Rev. 36, 1–369 (1996)
P. Wiesen, J. Kleffmann, R. Kurtenbach, K.H. Becker, Faraday Discuss. 100, 121–127 (1995)
X. Zhou, H. Gao, Y. He, G. Huang, S.B. Bertman, K. Civerolo, J. Schwab, Geophys. Res. Lett. 30, 23–2217 (2003)
B. Aumont, F. Chervier, S. Laval, Atmos. Environ. 37, 487–498 (2003)
M. Baumgartner, E. Bock, R. Conrad, Chemosphere 24, 1943–1960 (1992)
R. Bröske, J. Kleffmann, P. Wiesen, Atmos. Chem. Phys. Discuss. 3, 597–613 (2003)
J. Kleffmann, Chem. Phys. Chem. 8(8), 1137–1144 (2007)
F. Rohrer, B. Bohn, T. Brauers, D. Brüning, F.J. Johnen, A. Wahner, J. Kleffmann, Amos. Chem. Phys. 5, 2189–2201 (2005)
C. George, R.S. Strekowski, J. Kleffmann, K. Stemmler, M. Ammann, Faraday Discuss. 130, 195–210 (2005)
K. Stemmler, M. Ndour, Y. Elshorbany, J. Kleffmann, B. D’Anna, C. George, B. Bohn, M. Ammann, Atmos. Chem. Phys. 7, 16–4248 (2007)
M. Ammann, M. Kalberer, D.T. Jost, L. Tobler, E. Rössler, D. Piguet, H.W. Gäggeler, U. Baltensperger, Nature 395, 157–160 (1998)
M. Kalberer, M. Ammann, F. Arens, H.W. Gäggeler, U. Baltensper, J. Geophys. Res. 104, 13825–13832 (1999)
A. Gerecke, A. Thielmann, L. Gutzwiller, M.J. Rossi, Geophys. Res. Lett. 25, 2453–2456 (1998)
J. Kleffmann, K.H. Becker, M. Lackhoff, P. Wiesen, Phys. Chem. Chem. Phys. 1, 5443–5450 (1999)
J. Kleffmann, J. Heland, R. Kurtenbach, J. C. Lörzer, P. Wiesen, M. Ammann, L. Gutzwiller, M. Rodenas Garcia, M. Pons, K. Wirtz, V. Scheer, and R. Vogt, CMD Annual Rep., 177–180 (2000)
M. Ammann, F. Arens, L. Gutzwiller, U. Baltensperger, and H. W. Gäggeler, CMD Annual Rep. 140–143 (2000)
M.S. Akhter, A.R. Chughtai, D.M. Smith, J. Phys. Chem. 88, 5334–5342 (1984)
D.A. Hauglustaine, S. Madronuch, B.A. Ridley, J.G. Walega, C.A. Cantrell, R.E. Shetter, G. Hüber, J. Geophys. Res. 101, 14681–14696 (1996)
N.A. Saliba, H. Yang, B.J. Finlayson-Pitts, J. Phys. Chem. A. 105, 10339–10346 (2001)
M.S. Muñoza, M.J. Rossi, Phys. Chem. Chem. Phys. 4, 5110–5118 (2002)
D. Stadler, M.J. Rossi, Phys. Chem. Chem. Phys. 2, 5420–5429 (2000)
K. Tabor, L. Gutzwiller, M.J. Rossi, J. Phys. Chem. 98, 6172–6186 (1994)
M.S. Salgado, M.J. Rossi, Int J. Chem. Kinetic. 34(11), 620–631 (2002)
M.O. Andreae, T.W. Andreae, R.J. Ferek, H. Raemdonk, Sci. Total Environ. 36, 73–80 (1984)
A.D. Clarke, R.E. Weiss, R.J. Charlson, Sci. Total Environ. 36, 92–102 (1984)
A.D. Clarke, Aerosol Sci. Technol. 10, 161–171 (1989)
C.A. Rogaski, D.M. Golden, L.R. Williams, Geophys. Res. Lett. 24, 381–384 (1997)
C.A. Jornod, H.vd. Bergh, M.J. Rossi, Phys. Chem. Chem. Phys. 2, 5584–5593 (2000)
Å. Sjödin, Environ. Sci. Technol. 22, 1086–1089 (1988)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Indarto, A. Heterogeneous reactions of HONO formation from NO2 and HNO3: a review. Res Chem Intermed 38, 1029–1041 (2012). https://doi.org/10.1007/s11164-011-0439-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-011-0439-z