Abstract
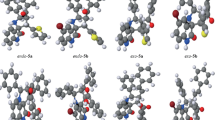
Spiro indane-1,3-dione compounds have been synthesized by 1,3-dipolar cycloaddition of ninhydrin, l-proline, and an alkene (either a chalcone or an (E)-β-arylnitrostyrene). All these reactions proceed with good yield and with high regioselectivity and stereoselectivity. The structures were studied by NMR spectroscopy, MS, and X-ray diffraction analysis. It was found that these two kinds of alkene lead to different regioselectivity. This study has provided information about the regioselectivity of 1,3-dipolar cycloaddition reactions: regioselectivity may be controlled by π–π stacking state; the order of stability of stacking of the Ar and EWG part with the indane-1,3-dione part is: benzoyl group > phenyl group > nitro group.




Similar content being viewed by others
References
G. Chen, H.P. He, J. Ding, X.J. Hao, Heterocyl. Commun. 15, 5–355 (2009)
S. Rehn, J. Bergman, B. Stensland, Eur. J. Org. Chem. 2, 413 (2004)
T. Okino, Y. Hoashi, T. Furukawa, X. Xu, Y. Takemoto, J. Am. Chem. Soc. 127, 119 (2005)
R.A. Amal, R. Raghunathan, M.R. SrideviKumari, N. Raman, Bioorg. Med. Chem. 11, 407 (2003)
R.S. Manian, J. Jayashankaran, S.S. Kumar, R. Raghunathan, Tetrahedron Lett. 47, 829 (2006)
N. Ono, H. Miyake, A. Kamimura, I. Hamamoto, R. Tamura, A. Kaji, Tetrahedron 41, 4013 (1985)
A.A.S. El-Ahl, Heteroatom Chem. 13, 324 (2002)
D. Fokas, W.J. Rvan, D.S. Casebier, D.L. Coffen, Tetrahedron Lett. 39, 2235 (1998)
F.D. Popp, J. Heter. Chem. 21, 1367 (1984)
R.T. Pardasani, P. Pardasani, V. Chaturvedi, S.K. Yadav, A. Saxena, I. Sharma, Heteroatom Chem. 14, 36 (2003)
M.R. Caira, F. Dumitrascu, C. Draghici, D. Dumitrescu, M. Cristea, Molecules 10, 360 (2005)
A. Javad, M. Ali Varasteh, S. Saeed, Ali Asadi, Tetrahedron Lett. 43, 9721 (2002)
A. Amal Raj, R. Raghunathan, Tetrahedron 59, 2907 (2003)
P. Mahalingam, R. Ragavachary, Synthetic Commun. 37, 2507 (2007)
Acknowledgment
This work was financially supported by the grants from National Science Foundation of China (no. 50874092) and Scientific Research Program Funded by Shaanxi Provincial Education Department (program no. 08JK413, 11JK0560).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, G., Zhang, J. & Wu, Y. Synthesis and regiochemistry of spiro indane-1,3-dione compounds. Res Chem Intermed 38, 413–420 (2012). https://doi.org/10.1007/s11164-011-0357-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-011-0357-0