Abstract
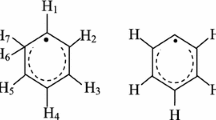
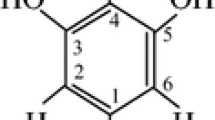
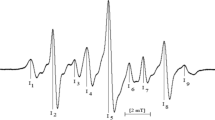
Radicals formed in γ-irradiated 1,3,5-trithiane (TT) and its three derivatives, α- and β-2,4,6-trimethyl-1,3,5-trithiane (α-TMT and β-TMT), and 2,4,6-trimethyl-2,4,6-triphenyl-1,3,5-trithiane (TMTPT), were studied by the electron paramagnetic resonance (EPR) method in the solid state. The sulfur radical cations (>S+•) were identified in all compounds at 77 K. In TT and its two derivatives, α-TMT and β-TMT, the >S+• decay via deprotonation-forming C-centered radicals. Further increase of temperature up to 293 K results in the appearance of thiyl-type radicals (RS•). In TMTPT, the >S+• are stable up to 250 K. They formed the intermolecularly three-electron-bonded dimeric radical cations (S∴S)+ while RS• radicals were not observed. Some of the radical assignments and their EPR parameters (g and a hyperfine splittings) obtained support from the DFT calculations.









Similar content being viewed by others
References
K.-D. Asmus, D. Bahnemann, M. Bonifacic, H.A. Gillis, Free radical oxidation of organic sulphur compounds in aqueous solution. Faraday Discuss. Chem. Soc. 63, 213–225 (1978)
K.-D. Asmus, H.A. Gillis, G.G. Teather, Radical cation complexes in the oxidation of organic di-, tri-, and tetrathia compounds in hydrocarbon solutions. J. Phys. Chem. 82, 2677–2682 (1978)
E. Janeba-Bartoszewicz, G.L. Hug, E. Andrzejewska, B. Marciniak, Photochemistry of 1, 3, 5-trithianes in solution. Steady-state and laser flash photolysis studies. J. Photochem. Photobiol. A. Chem 177, 17–23 (2006)
K.-D. Asmus, G.L. Hug, K. Bobrowski, Q.G. Mulazzani, B. Marciniak, Transients in the oxidative and H-Atom-induced degradation of 1, 3.5-Trithiane. Time-resolved studies in aqueous solution. J. Phys. Chem. A 110(929), 2–9300 (2006)
E. Janeba-Bartoszewicz, G.L. Hug, H. Kozubek, W. Urjasz, B. Marciniak, Photochemical reactions of β-2.4.6-trimethyl-1, 3, 5-trithiane in solution. J. Photochem. Photobiol. A. Chem 140(13), 3–139 (2001)
B. Marciniak, E. Andrzejewska, G.L. Hug, Photoreduction of benzophenone by 2, 4, 6-trimethyl-1, 3, 5-trithiane in solution. Laser flash photolysis study. J. Photochem. Photobiol. A 112, 21–28 (1998)
E. Andrzejewska, G.L. Hug, M. Andrzejewski, B. Marciniak, Trithianes as coinitiators in benzophenone-induced photopolymerizations. Macromolecules 32, 2173–2179 (1999)
J. Lando, V. Stanett, Synthesis of highly oriented polythiomethylene directly by radiation polymerization of crystalline trithiane. J. Polym. Sci. B 2, 375–378 (1964)
E. Andrzejewska, Photopolymerization kinetics of multifunctional monomers. Prog. Polym. Sci. 26, 605–665 (2001)
E. Andrzejewska, A. Zuk, J. Pietrzak, R. Krzyminiewski, ESR study of γ-irradiated 1, 3, 5-trithiane and its derivatives. J. Polym. Sci. 16, 2991–2996 (1978)
I.B. Douglas, H.W. R., The preparation of certain s-trithianes. J. Am. Chem. Soc. 73, 3507 (1951)
Z. El-Hewehi, D. Hempel, Zurkenntnis der s-trithiane. 2. Herstellung and eigeschaften der s-trithiane. J. Prakt. Chem. 21, 286–294 (1963)
A.D. Becke, Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993)
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheesemann, J. Montgomery, J. A., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennuci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. MArtin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, C. M., P. M. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, J. A. Pople, Gausian 03 (Rev. B.04) (2003)
F. Cloran, I. Carmichael, A.S. Serianni, Density functional calculations on disaccharide mimics: studies of molecular geometries and trans-0-glycosidic 3JCOCH and 3JCOCC spin couplings. J. Am. Chem. Soc. 121, 9843–9851 (1999)
R. Stenutz, I. Carmichael, G. Widmalm, A.S. Serrianni, Hydroxymethyl group conformation in saccharides; structural dependencies of 2JHH, 3JHH, 1JCH spin-spin coupling constants. J. Org. Chem. 67, 949–958 (2002)
F. Neese, Prediction of electron paramagnetic resonance g values using coupled perturbed Hartree-Fock and Kohn-Sham theory. J. Chem. Phys. 115, 11080–11096 (2001)
S. Kominami, A radical cation produced in a γ-irradiated single crystal of DL-methionine as studied by electron spin resonance and optical absorption spectroscopy. J. Phys. Chem. 76, 1729–1733 (1972)
K. Kawatsura, K. Ozawa, S. Kominami, K. Akasaka, H. Hatano, Optical and ESR studies N-acetyl DL-metionine single crystals γ-irradiated at low temperature. Radiat. Effects 22, 267–275 (1974)
G. Strzelczak, K. Bobrowski, H. Kozubek, J. Michalik, Electron spin resonance study of radiation-induced radicals in polycrystalline oligopeptides containing methionine. Nukleonika 45, 93–100 (2000)
C. Chatgilialoglu, M.P. Bertrand, C. Ferreri, Z.B. Alfassi, Sulfur-centered radicals in organic synthesis, S-centered Radicals, 311–354 (1999)
M.H. Champagne, M.W. Mullins, A.-O. Coulson, M.D. Sevilla, Electron spin resonanse evidence for intra- and intermolecular σσ* bonding in methionine radicals: relative stabilities of S–Cl, S–Br, S–N, and S–S three-electron bonds. J. Phys. Chem. 95, 6487–6493 (1991)
Acknowledgment
We would like to thank the Faculty of Chemistry in Adam Mickiewicz University (E.J.B., B.M.) and the Institute of Nuclear Chemistry (G.S., K.B.) for financial support. This work was partly supported by the Office of Basic Energy Sciences of the U.S. Department of Energy (I.C.). This paper is Document No. NDRL-4801 from the Notre Dame Radiation Laboratory.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Strzelczak, G., Janeba-Bartoszewicz, E., Carmichael, I. et al. Electron paramagnetic resonance (EPR) study of γ-radiation-induced radicals in 1,3,5-trithiane and its derivatives. Res Chem Intermed 35, 507–517 (2009). https://doi.org/10.1007/s11164-009-0054-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-009-0054-4