Abstract
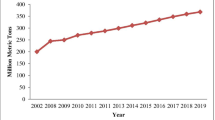
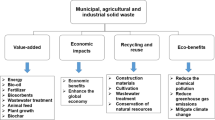
Reuse options for coal fly ash and coal bottom ash are reviewed in this paper. Although, significant quantities of coal fly ash and coal bottom ash are produced worldwide every year, less than 30 % of coal ash produced is reused. Coal ash is mainly reused in civil engineering applications such as road construction, embankments, construction materials, geo-polymer applications and in cement production. Other potential reuse options for coal ash include applications such as glass ceramics, water and wastewater treatment, agriculture as well as for making high value products (e.g. telescope mirrors, break-liners, fire proof products etc.). Considering that only a small fraction of coal ash is reused, other reuse options for commercial applications need to be explored.

Similar content being viewed by others
Abbreviations
- ASTM:
-
American Society of Testing of Materials
- CBA:
-
Coal bottom ash
- CFA:
-
Coal fly ash
- DNA:
-
Deoxyribonucleic acid
- EU:
-
European Union
- FGD:
-
Flue gas desulfurization
- GBA:
-
Ground bottom ash
- HeCB:
-
Heptachloro biphenyl
- LOI:
-
Loss on ignition
- OPC:
-
Ordinary Portland cement
- PAH:
-
Polycyclic aromatic hydrocarbon
- PCB:
-
Polychlorinated biphenyl
- TCB:
-
Tri chloro biphenyl
- TOC:
-
Total organic carbon
- TPPs:
-
Thermal power plants
- TW:
-
Tinacal ore waste
- ZFA:
-
Zeolited fly ash
- USA:
-
United States of America
References
ACAA (2010) 2010 coal combustion product (CCP) Production and use survey report. American Coal Ash Association. http://acaa.affiniscape.com/associations/8003/files/2010_CCP_Survey_FINAL_102011.pdf. Accessed 20.02.2012
Agyei NM, Strydom CA, Potgieter JH (2002) The removal of phosphate ions from aqueous solution by fly ash, slag, ordinary Portland cement and related blends. Cem Concr Res 32(12):1889–1897. doi:10.1016/s0008-8846(02)00888-8
Ahmaruzzaman M (2008) Adsorption of phenolic compounds on low-cost adsorbents: a review. Adv Colloid Interface 143(1–2):48–67
Ahmaruzzaman M (2010) A review on the utilization of fly ash. Prog Energy Combust Sci 36(3):327–363. doi:10.1016/j.pecs.2009.11.003
Arenillas A, Smith KM, Drage TC, Snape CE (2005) CO2 capture using some fly ash-derived carbon materials. Fuel 84(17):2204–2210. doi:10.1016/j.fuel.2005.04.003
Asokan P, Saxena M, Asolekar SR (2005) Coal combustion residues—environmental implications and recycling potentials. Resour Conserv Recycl 43(3):239–262. doi:10.1016/j.resconrec.2004.06.003
Ayala J, Blanco F, García P, Rodriguez P, Sancho J (1998) Asturian fly ash as a heavy metals removal material. Fuel 77(11):1147–1154. doi:10.1016/s0016-2361(98)00027-1
Baba A, Kaya A (2004) Leaching characteristics of solid wastes from thermal power plants of western Turkey and comparison of toxicity methodologies. J Environ Manag 73(3):199–207. doi:10.1016/j.jenvman.2004.06.005
Baba A, Gurdal G, Sengunalp F, Ozay O (2008) Effects of leachant temperature and pH on leachability of metals from fly ash. A case study: can thermal power plant, province of Canakkale, Turkey. Environ Monit Assess 139(1):287–298
Barbieri L, Lancellotti I, Manfredini T, Ignasi Q, Rincon JM, Romero M (1999) Design, obtainment and properties of glasses and glass-ceramics from coal fly ash. Fuel 78(2):271–276. doi:10.1016/s0016-2361(98)00134-3
Bashkin VN, Wongyai K (2002) Environmental fluxes of arsenic from lignite mining and power generation in northern Thailand. Environ Geol 41(8):883–888
Bhangare RC, Ajmal PY, Sahu SK, Pandit GG, Puranik VD (2011) Distribution of trace elements in coal and combustion residues from five thermal power plants in India. Int J Coal Geol. doi:10.1016/j.coal.2011.03.008
Bhargava R, Mathur R, Khanna P (1974) Removal of detergent from wastewater by adsorption on fly ash. Indian J Environ Health 16(2):109–120
Brigden K, Santillo D, Stringer R (2002) Hazardous emissions from Thai coal-fired power plants. Toxic and potentially toxic elements in fly ashes collected from the Mae Moh and Thai Petrochemical industry coal-fired power plants in Thailand, 2002. Greenpeace Research Laboratories, Department of Biological Sciences, University of Exeter, Exeter
Bruce RB, Kuntze RA (1983) Sag-resistant gypsum board containing coal fly ash and method for making same. US Patent No 4,403,006, 6 Sept 1983
Cao D-z, Selic E, Herbell J-D (2008) Utilization of fly ash from coal-fired power plants in China. J Zhejiang Univ Sci A 9(5):681–687. doi:10.1631/jzus.A072163
Chakraborty R, Mukherjee A (2009) Mutagenicity and genotoxicity of coal fly ash water leachate. Ecotoxicol Environ Saf 72(3):838–842. doi:10.1016/j.ecoenv.2008.09.023
Chaturvedi A, Yadava K, Pathak K, Singh V (1990) Defluoridation of water by adsorption on fly ash. Water Air Soil Pollut 49(1):51–61
Chaulia PK, Biswajit R, Maity SN (2009) Utilisation of flyash as gainful resource material for green brick making. Res J Chem Environ 13(4):10–12
Chen QY, Tyrer M, Hills CD, Yang XM, Carey P (2009) Immobilisation of heavy metal in cement-based solidification/stabilisation: a review. Waste Manag 29(1):390–403. doi:10.1016/j.wasman.2008.01.019
Chen XY, Wendell K, Zhu J, Li JL, Yu X, Zhang Z (2012) Synthesis of nano-zeolite from coal fly ash and its potential for nutrient sequestration from anaerobically digested swine wastewater. Bioresour Technol 110:79–85
Cheriaf M, Rocha JC, Péra J (1999) Pozzolanic properties of pulverized coal combustion bottom ash. Cem Concr Res 29(9):1387–1391. doi:10.1016/s0008-8846(99)00098-8
Chindaprasirt P, Jaturapitakkul C, Chalee W, Rattanasak U (2009) Comparative study on the characteristics of fly ash and bottom ash geopolymers. Waste Manag 29(2):539–543. doi:10.1016/j.wasman.2008.06.023
Cho H, Oh D, Kim K (2005) A study on removal characteristics of heavy metals from aqueous solution by fly ash. J Hazard Mater 127(1–3):187–195
Choi SK, Lee S, Song YK, Moon HS (2002) Leaching characteristics of selected Korean fly ashes and its implications for the groundwater composition near the ash disposal mound. Fuel 81(8):1083–1090. doi:10.1016/s0016-2361(02)00006-6
Cokca E, Yilmaz Z (2004) Use of rubber and bentonite added fly ash as a liner material. Waste Manag 24(2):153–164. doi:10.1016/j.wasman.2003.10.004
Collot A-G (2006) Matching gasification technologies to coal properties. Int J Coal Geol 65(3–4):191–212. doi:10.1016/j.coal.2005.05.003
Conner JR, Hoeffner SL (1998) A critical review of stabilization/solidification technology. Crit Rev Environ Sci Technol 28(4):397–462
Cumpston B, Shadman F, Risbud S (1992) Utilization of coal-ash minerals for technological ceramics. J Mater Sci 27(7):1781–1784. doi:10.1007/bf01107204
Daci MN, Daci NM, Zeneli L, Gashi S, Hoxha D (2011) Coal ash as adsorbent for heavy metal ions in standard solutions, industrial wastewater and streams. Ecohydrol Hydrobiol 11(1):129–132
Davies L (2011) Beyond Fukushima: disasters, nuclear energy, and energy law. BYU Law Rev 2011:1937
Dermatas D, Meng X (2003) Utilization of fly ash for stabilization/solidification of heavy metal contaminated soils. Eng Geol 70(3–4):377–394. doi:10.1016/s0013-7952(03)00105-4
Dewangan P, Pradhan M, Kishore N (2010) Utilisation of fly ash as a structural fill material for safe and sustainable development: need of the hour. IME J 134–139
Diamadopoulos E, Ioannidis S, Sakellaropoulos GP (1993) As(V) removal from aqueous solutions by fly ash. Water Res 27(12):1773–1777. doi:10.1016/0043-1354(93)90116-y
Dinçer AR, Günes Y, Karakaya N (2007) Coal-based bottom ash (CBBA) waste material as adsorbent for removal of textile dyestuffs from aqueous solution. J Hazard Mater 141(3):529–535. doi:10.1016/j.jhazmat.2006.07.064
Dizge N, Aydiner C, Demirbas E, Kobya M, Kara S (2008) Adsorption of reactive dyes from aqueous solutions by fly ash: kinetic and equilibrium studies. J Hazard Mater 150(3):737–746. doi:10.1016/j.jhazmat.2007.05.027
Dutta BK, Khanra S, Mallick D (2009) Leaching of elements from coal fly ash: assessment of its potential for use in filling abandoned coal mines. Fuel 88(7):1314–1323. doi:10.1016/j.fuel.2009.01.005
ECOBA (2008) Production and utilisation of CCPs in 2008 in Europe (EU 15). European Coal Combustion Products Association. www.ecoba.org. Accessed 02.20.2012
EGAT (2010) Personal communication with Mae Moh Power Plant, Thailand. Discussion notes with Mae Moh Authrity during site visit made on 23–24 September 2011
EPRI (1996) Coal ash: its origin, disposal, use, and potential health issues. EPRI TR-106516 (Section B)
Fairbrother A, Bigham G, Pietari J, Mohsen F (2010) Coal ash: hazard, waste, or resources? Newsl Expon Environ Eco Sci Pract 1:1–11
Fang Z, Gesser H (1996) Recovery of gallium from coal fly ash. Hydrometallurgy 41(2–3):187–200
Fisher GL, Chang D, Brummer M (1976) Fly ash collected from electrostatic precipitators: microcrystalline structures and the mystery of the spheres. Science 192(4239):553
Francis AA, Rawlings RD, Sweeney R, Boccaccini AR (2004) Crystallization kinetic of glass particles prepared from a mixture of coal ash and soda-lime cullet glass. J Non Cryst Solids 333(2):187–193. doi:10.1016/j.jnoncrysol.2003.09.048
Furlong T, Hearne J (1994) Process for producing solid bricks from fly ash, bottom ash, lime, gypsum, and calcium carbonate. Google Patents
Gal M, Hollis J, Keren R (1988) Boron release and sorption by fly ash as affected by pH and particle size. J Environ Qual 17(2):181–184
Geetha S, Ramamurthy K (2010) Environmental friendly technology of cold-bonded bottom ash aggregate manufacture through chemical activation. J Clean Prod 18(15):1563–1569. doi:10.1016/j.jclepro.2010.06.006
Giergiczny Z, Król A (2008) Immobilization of heavy metals (Pb, Cu, Cr, Zn, Cd, Mn) in the mineral additions containing concrete composites. J Hazard Mater 160(2–3):247–255. doi:10.1016/j.jhazmat.2008.03.007
Gitari WM, Petrik LF, Key DL, Okujeni C (2010) Interaction of acid mine drainage with Ordinary Portland Cement blended solid residues generated from active treatment of acid mine drainage with coal fly ash. J Environ Sci Health, Part A 46(2):117–137
Gorme JB, Kim SS, Kim YT (2010) Characterization of bottom ash as an adsorbent of lead from aqueous solutions. Environ Eng Res 15(4):207–213
Gupta G, Torres N (1998) Use of fly ash in reducing toxicity of and heavy metals in wastewater effluent. J Hazard Mater 57(1–3):243–248. doi:10.1016/s0304-3894(97)00093-9
Gupta VK, Mittal A, Krishnan L, Gajbe V (2004) Adsorption kinetics and column operations for the removal and recovery of malachite green from wastewater using bottom ash. Sep Purif Technol 40(1):87–96. doi:10.1016/j.seppur.2004.01.008
Gupta VK, Mittal A, Krishnan L, Mittal J (2006) Adsorption treatment and recovery of the hazardous dye, Brilliant Blue FCF, over bottom ash and de-oiled soya. J Colloid Interface Sci 293(1):16–26. doi:10.1016/j.jcis.2005.06.021
Haibin L, Zhenling L (2010) Recycling utilization patterns of coal mining waste in China. Resour Conserv Recycl 54(12):1331–1340. doi:10.1016/j.resconrec.2010.05.005
Hansen Y, Notten PJ, Petrie JG (2002) The environmental impact of ash management in coal-based power generation. Appl Geochem 17(8):1131–1141. doi:10.1016/s0883-2927(02)00013-6
Horiuchi S, Kawaguchi M, Yasuhara K (2000) Effective use of fly ash slurry as fill material. J Hazard Mater 76(2–3):301–337. doi:10.1016/s0304-3894(00)00205-3
Hsu T-C (2008) Adsorption of an acid dye onto coal fly ash. Fuel 87(13–14):3040–3045. doi:10.1016/j.fuel.2008.03.026
Hsu TC, Yu CC, Yeh CM (2008) Adsorption of Cu 2 + from water using raw and modified coal fly ashes. Fuel 87(7):1355–1359
Huang WH (1990) The use of bottom ash in highway embankments, subgrades, and subbases. Indiana Department of Transportation and Purdue University, West Lafayette
Hui KS, Chao CYH (2006) Pure, single phase, high crystalline, chamfered-edge zeolite 4A synthesized from coal fly ash for use as a builder in detergents. J Hazard Mater 137(1):401–409. doi:10.1016/j.jhazmat.2006.02.014
Hui K, Chao C, Kot S (2005) Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash. J Hazard Mater 127(1):89–101
Hui K, Hui K, Lee SK (2009) A Novel and green approach to produce nano-porous materials zeolite A and MCM-41 from coal fly ash and their applications in environmental protection. Int J Chem Biol Eng 2:4
IEA (2012) Key world energy statistics. International Energy Agency. www.iea.org/publications/freepublications/publication/kwes.pdf. Accessed 05.10.2013
Iyer R (2002) The surface chemistry of leaching coal fly ash. J Hazard Mater 93(3):321–329. doi:10.1016/s0304-3894(02)00049-3
Izquierdo M, Querol X (2012) Leaching behaviour of elements from coal combustion fly ash: an overview. Int J Coal Geol 94:54–66. doi:10.1016/j.coal.2011.10.006
Izquierdo M, Querol X, Davidovits J, Antenucci D, Nugteren H, Fernández-Pereira C (2009) Coal fly ash-slag-based geopolymers: microstructure and metal leaching. J Hazard Mater 166(1):561–566
Janos P, Wildnerová M, Loucka T (2002) Leaching of metals from fly ashes in the presence of complexing agents. Waste Manag 22(7):783–789. doi:10.1016/s0956-053x(02)00039-9
Janos P, Buchtová H, Rýznarová M (2003) Sorption of dyes from aqueous solutions onto fly ash. Water Res 37(20):4938–4944. doi:10.1016/j.watres.2003.08.011
Jaturapitakkul C, Cheerarot R (2003) Development of bottom ash as pozzolanic material. J Mater Civ Eng 15:48
Jayaranjan M, Annachhatre AP (2012) Precipitation of heavy metals from coal ash leachate using biogenic hydrogen sulfide generated from FGD gypsum. Water Sci Technol J Int Assoc Water Pollut Res 67(2):311–318
Jones MR, McCarthy A (2005) Utilising unprocessed low-lime coal fly ash in foamed concrete. Fuel 84(11):1398–1409. doi:10.1016/j.fuel.2004.09.030
Juan R, Hernández S, Andrés JM, Ruiz C (2007) Synthesis of granular zeolitic materials with high cation exchange capacity from agglomerated coal fly ash. Fuel 86(12–13):1811–1821. doi:10.1016/j.fuel.2007.01.011
Kara A, Kurama H, Kara Y, Kurama S (2004) Utilization of coal combustion fly ash in terracotta bodies. Key Eng Mater 264:2513–2516
Kara S, Aydiner C, Demirbas E, Kobya M, Dizge N (2007) Modeling the effects of adsorbent dose and particle size on the adsorption of reactive textile dyes by fly ash. Desalination 212(1–3):282–293
Kayabal K, Bulus G (2000) The usability of bottom ash as an engineering material when amended with different matrices. Eng Geol 56(3–4):293–303. doi:10.1016/s0013-7952(99)00097-6
Kim B (2003) Properties of coal ash mixtures and their use in highway embankments. Civil engineering. PhD. Purdue University, Ann Arbor, p 240
Kim AG, Hesbach P (2009) Comparison of fly ash leaching methods. Fuel 88(5):926–937. doi:10.1016/j.fuel.2008.11.013
Kim B, Prezzi M, Salgado R (2005) Geotechnical properties of fly and bottom ash mixtures for use in highway embankments. J Geotech Geoenviron Eng 131:914
Kimura N, Omata K, Kiga T, Takano S, Shikisima S (1995) The characteristics of pulverized coal combustion in O2/CO2 mixtures for CO2 recovery. Energy Convers Manag 36(6–9):805–808. doi:10.1016/0196-8904(95)00126-X
Kizgut S, Cuhadaroglu D, Samanli S (2010) Stirred grinding of coal bottom ash to be evaluated as a cement additive. Energy Source Part A 32(16):1529–1539. doi:10.1080/15567030902780378
Kniess CT, de Lima JC, Prates PB, Kuhnen NC, Riella HG (2007) Dilithium dialuminium trisilicate phase obtained using coal bottom ash. J Non Cryst Solids 353(52–54):4819–4822. doi:10.1016/j.jnoncrysol.2007.06.047
Kolay P, Singh D (2001) Physical, chemical, mineralogical, and thermal properties of cenospheres from an ash lagoon. Cem Concr Res 31(4):539–542
Korcak R (1998) Agricultural uses of coal combustion byproducts. In: Agricultural uses of municipal, animal and industrial byproducts. USDA-ARS Conservation Res Rep 44:103–119
Koukouzas N, Vasilatos C, Itskos G, Mitsis I, Moutsatsou A (2010) Removal of heavy metals from wastewater using CFB-coal fly ash zeolitic materials. J Hazard Mater 173(1):581–588
Kraus RN, Chun Y, Ramme BW, Singh SS (2003) Properties of field manufactured cast-concrete products utilizing recycled materials. J Mater Civ Eng 15:400
Kula I, Olgun A, Sevinc V, Erdogan Y (2002) An investigation on the use of tincal ore waste, fly ash, and coal bottom ash as Portland cement replacement materials. Cem Concr Res 32(2):227–232. doi:10.1016/s0008-8846(01)00661-5
Kumar S, Patil CB (2006) Estimation of resource savings due to fly ash utilization in road construction. Resour Conserv Recycl 48(2):125–140. doi:10.1016/j.resconrec.2006.01.002
Kumar S, Stewart J (2003a) Evaluation of Illinois pulverized coal combustion dry bottom ash for use in geotechnical engineering applications. J Energy Eng 129:42
Kumar S, Stewart J (2003b) Utilization of Illinois PCC dry bottom ash for compacted landfill barriers. Soil Sediment Contam Int J 12(3):401–415
Kumpiene J, Lagerkvist A, Maurice C (2007) Stabilization of Pb- and Cu-contaminated soil using coal fly ash and peat. Environ Pollut 145(1):365–373. doi:10.1016/j.envpol.2006.01.037
Kurama H, Kaya M (2008) Usage of coal combustion bottom ash in concrete mixture. Constr Build Mater 22(9):1922–1928. doi:10.1016/j.conbuildmat.2007.07.008
Lav AH, Lav MA, Goktepe AB (2006) Analysis and design of a stabilized fly ash as pavement base material. Fuel 85(16):2359–2370. doi:10.1016/j.fuel.2006.05.017
Lee JM, Kim DW, Kim JS (2011) Characteristics of co-combustion of anthracite with bituminous coal in a 200-MWe circulating fluidized bed boiler. Energy 36(9):5703–5709. doi:10.1016/j.energy.2011.06.051
Leonards GA, Bailey B (1982) Pulverized coal ash as structural fill. J Geotech Eng Div Am Soc Civ Eng 108:517–531
Levandowski J, Kalkreuth W (2009) Chemical and petrographical characterization of feed coal, fly ash and bottom ash from the Figueira Power Plant, Paraná, Brazil. Int J Coal Geol 77(3–4):269–281. doi:10.1016/j.coal.2008.05.005
Lin CY, Yang DH (2002) Removal of pollutants from wastewater by coal bottom ash. J Environ Sci Health, Part A 37(8):1509–1522
Lopez-Anton M, Diaz-Somoano M, Fierro J, Martinez-Tarazona M (2007) Retention of arsenic and selenium compounds present in coal combustion and gasification flue gases using activated carbons. Fuel Process Technol 88(8):799–805
Manz OE (1999) Coal fly ash: a retrospective and future look. Fuel 78(2):133–136. doi:10.1016/s0016-2361(98)00148-3
Mathieu JL, Gadgil AJ, Addy SEA, Kowolik K (2010) Arsenic remediation of drinking water using iron-oxide coated coal bottom ash. J Environ Sci Health, Part A 45(11):1446–1460
Matjie RH, Bunt JR, van Heerden JHP (2005) Extraction of alumina from coal fly ash generated from a selected low rank bituminous South African coal. Miner Eng 18(3):299–310. doi:10.1016/j.mineng.2004.06.013
McCarthy MJ, Dhir RK (1999) Towards maximising the use of fly ash as a binder. Fuel 78(2):121–132. doi:10.1016/s0016-2361(98)00151-3
Mehta P (1998) Role of pozzolanic and cementious material in sustainable development of the concrete industry. Spec Publ 178:1–20
Mittal A, Kurup L, Gupta VK (2005) Use of waste materials—bottom ash and de-oiled soya, as potential adsorbents for the removal of Amaranth from aqueous solutions. J Hazard Mater 117(2–3):171–178. doi:10.1016/j.jhazmat.2004.09.016
Mohan D, Singh KP, Singh G, Kumar K (2002) Removal of dyes from wastewater using fly ash, a low-cost adsorbent. Ind Eng Chem Res 41(15):3688–3695
Mohanty S, Chugh YP (2007) Development of fly ash-based automotive brake lining. Tribol Int 40(7):1217–1224. doi:10.1016/j.triboint.2007.01.005
Mondragon F, Rincon F, Sierra L, Escobar J, Ramirez J, Fernandez J (1990) New perspectives for coal ash utilization: synthesis of zeolitic materials. Fuel 69(2):263–266. doi:10.1016/0016-2361(90)90187-u
Montagnaro F, Santoro L (2009) Reuse of coal combustion ashes as dyes and heavy metal adsorbents: effect of sieving and demineralization on waste properties and adsorption capacity. Chem Eng J 150(1):174–180
Montes-Hernandez G, Pérez-López R, Renard F, Nieto JM, Charlet L (2009) Mineral sequestration of CO2 by aqueous carbonation of coal combustion fly-ash. J Hazard Mater 161(2–3):1347–1354. doi:10.1016/j.jhazmat.2008.04.104
Moulton LK (1973) Bottom ash and boiler slag. In: Proceedings of the third international ash utilization symposium. Sponsored by National Coal Association, Edison Electric Institute, American Public Power Association, National Ash Association, and Bureau of Mines, Pittsburgh, 13–14 March 1973
Moulton LK, Seals RK, Anderson DA (1973) Utilization of ash from coal-burning power plants in highway construction. Highw Res Rec 430:26–39
Mukhtar S, Kenimer AL, Sadaka SS, Mathis JG (2003) Evaluation of bottom ash and composted manure blends as a soil amendment material. Bioresour Technol 89(3):217–228. doi:10.1016/S0960-8524(03)00085-3
Muñoz MI, Aller AJ (2012) Chemical modification of coal fly ash for the retention of low levels of lead from aqueous solutions. Fuel. doi:10.1016/j.fuel.2012.06.042
Naik TR, Kraus RN, Chun Y, Botha FD (2005) Cast-concrete products made with FBC ash and wet-collected coal-ash. J Mater Civ Eng 17:659
Nathan Y, Dvorachek M, Pelly I, Mimran U (1999) Characterization of coal fly ash from Israel. Fuel 78(2):205–213
Nemade P, Alappat B (2002) Removal of fluorides from water using low cost adsorbents. Water Supply 2(1):311–317
Nollet H, Roels M, Lutgen P, Van der Meeren P, Verstraete W (2003) Removal of PCBs from wastewater using fly ash. Chemosphere 53(6):655–665. doi:10.1016/s0045-6535(03)00517-4
Ohtake T, Uchida K, Ikazaki F, Kawamura M, Ohkubo T, Kamiya K (1991) Synthesis of mullite from fly ash and alumina powder mixture. J Ceram Soc Jpn 99(1147):239–243
Olgun A, Erdogan Y, Ayhan Y, Zeybek B (2005) Development of ceramic tiles from coal fly ash and tincal ore waste. Ceram Int 31(1):153–158. doi:10.1016/j.ceramint.2004.04.007
Palumbo AV, McCarthy JF, Amonette JE, Fisher LS, Wullschleger SD, Daniels WL (2004) Prospects for enhancing carbon sequestration and reclamation of degraded lands with fossil-fuel combustion by-products. Adv Environ Res 8(3–4):425–438. doi:10.1016/s1093-0191(02)00124-7
Pandey VC, Singh JS, Singh RP, Singh N, Yunus M (2011) Arsenic hazards in coal fly ash and its fate in Indian scenario. Resour Conserv Recycl 55(9):819–835
Papandreou A, Stournaras CJ, Panias D (2007) Copper and cadmium adsorption on pellets made from fired coal fly ash. J Hazard Mater 148(3):538–547. doi:10.1016/j.jhazmat.2007.03.020
Papandreou AD, Stournaras CJ, Panias D, Paspaliaris I (2011) Adsorption of Pb(II), Zn(II) and Cr(III) on coal fly ash porous pellets. Miner Eng doi:10.1016/j.mineng.2011.07.016
Peng F, Liang K, Hu A, Shao H (2004) Nano-crystal glass-ceramics obtained by crystallization of vitrified coal fly ash. Fuel 83(14–15):1973–1977. doi:10.1016/j.fuel.2004.04.008
Pengthamkeerati P, Satapanajaru T, Chularuengoaksorn P (2008) Chemical modification of coal fly ash for the removal of phosphate from aqueous solution. Fuel 87(12):2469–2476
Pereira CF, RodrIguez-Piñero M, Vale J (2001) Solidification/stabilization of electric arc furnace dust using coal fly ash: analysis of the stabilization process. J Hazard Mater 82(2):183–195. doi:10.1016/s0304-3894(00)00359-9
Pimraksa K, Wilhelm M, Wruss W (2000) A new approach to the production of bricks made of 100% fly ash. In: International ash utilization symposium, Centre for Applied Energy Research, University of Kentucky, p 84
Pires M, Querol X (2004) Characterization of Candiota (South Brazil) coal and combustion by-product. Int J Coal Geol 60(1):57–72. doi:10.1016/j.coal.2004.04.003
Polat H, Vengosh A, Pankratov I, Polat M (2004) A new methodology for removal of boron from water by coal and fly ash. Desalination 164(2):173–188. doi:10.1016/s0011-9164(04)00176-6
Popovic A, Djordjevic D (2009) pH-dependent leaching of dump coal ash—retrospective environmental analysis. Energy Sources Part A Recovery Util Environ Eff 31(17):1553–1560
Popovic A, Djordjevic D, Polic P (2001) Trace and major element pollution originating from coal ash suspension and transport processes. Environ Int 26(4):251–255. doi:10.1016/s0160-4120(00)00114-8
Prasad B, Mondal K (2008) The impact of filling an abandoned open cast mine with fly ash on ground water quality: a case study. Miner Water Environ 27(1):40–45. doi:10.1007/s10230-007-0021-5
Prasad B, Sangita K, Tewary B (2011) Reducing the hardness of mine water using transformed fly ash. Mine Water Environ 30(1):61–66. doi:10.1007/s10230-010-0130-4
Prezzi M, Kim B (2008) Compaction characteristics and corrosivity of Indiana class-F fly and bottom ash mixtures. Constr Build Mater 22(4):694–702. doi:10.1016/j.conbuildmat.2006.09.007
Querol X, Moreno N, Umana JC, Alastuey A, Hernández E, López-Soler A, Plana F (2002) Synthesis of zeolites from coal fly ash: an overview. Int J Coal Geol 50(1):413–423
Ram LC, Masto RE (2010) An appraisal of the potential use of fly ash for reclaiming coal mine spoil. J Environ Manag 91(3):603–617. doi:10.1016/j.jenvman.2009.10.004
Ram L, Srivastava N, Jha S, Sinha A, Masto R, Selvi V (2007) Management of lignite fly ash for improving soil fertility and crop productivity. Environ Manag 40(3):438–452. doi:10.1007/s00267-006-0126-9
Rifa A, Yasufuku N, Omine K, Tsuji K (2009) Experimental study of coal ash utilization for road application on soft soil. Paper presented at the international joint symposium on geodisaster prevention and geoenvironment in Asia JS-Fukuoka
Russell NV, Méndez LB, Wigley F, Williamson J (2002) Ash deposition of a Spanish anthracite: effects of included and excluded mineral matter. Fuel 81(5):657–663. doi:10.1016/s0016-2361(01)00155-7
Sampaolo A, Relini G (1994) Coal ash for artificial habitats in Italy. Bull Mar Sci 55(2–3):1277–1294
Sathonsaowaphak A, Chindaprasirt P, Pimraksa K (2009) Workability and strength of lignite bottom ash geopolymer mortar. J Hazard Mater 168(1):44–50. doi:10.1016/j.jhazmat.2009.01.120
Sato A, Nishimoto S (2001) Effective reuse of coal ash as civil engineering material. In: Proceedings of the world of coal ash conference, Lexington, April, pp 11–15
Saxena M, Prabakhar J (2000) Emerging technologies for third millennium on wood substitute and paint from coal ash, vol 2, pp 26–28
Sell N, McIntosh T, Severance C, Peterson A (1989) The agronomic landspreading of coal bottom ash: using a regulated solid waste as a resource. Resour Conserv Recycl 2(2):119–129. doi:10.1016/0921-3449(89)90019-0
Sen AK, De AK (1987) Adsorption of mercury(II) by coal fly ash. Water Res 21(8):885–888. doi:10.1016/s0043-1354(87)80003-9
Sharma S, Fulekar M, Jayalakshmi C, Straub CP (1989) Fly ash dynamics in soil-water systems. Crit Rev Environ Sci Technol 19(3):251–275
Shih W-H, Chang H-L (1996) Conversion of fly ash into zeolites for ion-exchange applications. Mater Lett 28(4–6):263–268. doi:10.1016/0167-577x(96)00064-x
Silva LFO, Ward CR, Hower JC, Izquierdo M, Waanders F (2010) Mineralogy and leaching characteristics of coal ash from a major Brazilian power plant. Coal Combust Gasif Prod 2:51–65
Singh N (2009) Adsorption of herbicides on coal fly ash from aqueous solutions. J Hazard Mater 168(1):233–237
Singh RP, Gupta AK, Ibrahim MH, Mittal AK (2010) Coal fly ash utilization in agriculture: its potential benefits and risks. Rev Environ Sci Bio 9(4):345–358. doi:10.1007/s11157-010-9218-3
Sokolar R, Smetanova L (2010) Dry pressed ceramic tiles based on fly ash-clay body: influence of fly ash granulometry and pentasodium triphosphate addition. Ceram Int 36(1):215–221. doi:10.1016/j.ceramint.2009.07.009
Solis-Guzman J, Arenas CG, Marrero M, Leiva C, Arenas LFV (2011) High fire resistance in blocks containing coal combustion fly ashes and bottom ash. Waste Manag 31(8):1783–1789. doi:10.1016/j.wasman.2011.03.017
Soong Y, Fauth DL, Howard BH, Jones JR, Harrison DK, Goodman AL, Gray ML, Frommell EA (2006) CO2 sequestration with brine solution and fly ashes. Energy Convers Manag 47(13–14):1676–1685. doi:10.1016/j.enconman.2005.10.021
Steenbruggen G, Hollman GG (1998) The synthesis of zeolites from fly ash and the properties of the zeolite products. J Geochem Explor 62(1–3):305–309. doi:10.1016/s0375-6742(97)00066-6
Su T, Wang J (2011) Modeling batch leaching behavior of arsenic and selenium from bituminous coal fly ashes. Chemosphere 85(8):1368–1374
Sun W-l, Qu Y-z, Yu Q, Ni J-r (2008) Adsorption of organic pollutants from coking and papermaking wastewaters by bottom ash. J Hazard Mater 154(1–3):595–601. doi:10.1016/j.jhazmat.2007.10.063
Suwanvitaya P, Wattanachai P (2006) Comparison of metals leaching from Mortar with Mae Moh and Calaca bottom ashes as sand replacement. http://www.cv.titech.ac.jp/~jsps/activity_report/2005/Patcharaporn.pdf
Taeyoon L (2011) Leaching characteristics of bottom ash from coal fired electric generating plants, and waste tire; individually and mixtures when used as construction site fill materials. Waste Manag 31(2):246–252. doi:10.1016/j.wasman.2010.10.010
Theis TL, Gardner KH (1990) Environmental assessment of ash disposal. Crit Rev Environ Sci Technol 20(1):21–42
Twardowska I, Szczepanska J (2002) Solid waste: terminological and long-term environmental risk assessment problems exemplified in a power plant fly ash study. Sci Total Environ 285(1–3):29–51. doi:10.1016/s0048-9697(01)00893-2
Uçurum M, Toraman Ö, Depci T, Yo urtçuo lu E (2011) A study on characterization and use of flotation to separate unburned carbon in bottom ash from Çayirhan power plant. Energy Sources Part A Recovery Util Environ Eff 33(6):562–574
Ugurlu A, Salman B (1998) Phosphorus removal by fly ash. Environ Int 24(8):911–918. doi:10.1016/s0160-4120(98)00079-8
USEPA (2011) EPA promoted the use of coal ash products with incomplete risk information (trans: U.S. Environmental Protection Agency OoIG)
USEPA (2013) Waste-non hazardous wastes-industrial wastes. http://www.epa.gov/wastes/nonhaz/industrial/special/fossil/ccr-rule/ccrfaq.htm#3. Accessed 16.01.2014
Vassilev SV, Vassileva CG, Karayigit AI, Bulut Y, Alastuey A, Querol X (2005) Phase–mineral and chemical composition of composite samples from feed coals, bottom ashes and fly ashes at the Soma power station, Turkey. Int J Coal Geol 61(1–2):35–63. doi:10.1016/j.coal.2004.06.004
Vilches LF, Fernández Pereira C, Olivares del Valle J, Rodríguez Piñero M, Vale J (2002) Development of new fire proof products made from coal fly ash: the CEFYR project. J Chem Technol Biotechnol 77(3):361–366
Vilches LF, Fernández-Pereira C, Olivares del Valle J, Vale J (2003) Recycling potential of coal fly ash and titanium waste as new fireproof products. Chem Eng J 95(1–3):155–161. doi:10.1016/s1385-8947(03)00099-8
Viraraghavan T, Dronamraju M (1992) Utilization of coal ash in water pollution control. Int J Environ Stud 40(1):79–85
Vivoda V (2012) Japan’s energy security predicament post-Fukushima. Energy Policy 46:135–143
Vujić J, Antić DP, Vukmirović Z (2012) Environmental impact and cost analysis of coal versus nuclear power: the US case. Energy 45(1):31–42
Wang S, Boyjoo Y, Choueib A, Zhu ZH (2005) Removal of dyes from aqueous solution using fly ash and red mud. Water Res 39(1):129–138. doi:10.1016/j.watres.2004.09.011
Wearing C, Birch C, Nairn J (2004) An assessment of Tarong bottom ash for use on agricultural soils. Dev Chem Eng Miner Process 12(5–6):531–543
Wee HL, Wu H, Zhang D-k, French D (2005) The effect of combustion conditions on mineral matter transformation and ash deposition in a utility boiler fired with a sub-bituminous coal. Proc Combust Inst 30(2):2981–2989. doi:10.1016/j.proci.2004.08.059
Woolard C, Petrus K, Van der Horst M (2000) The use of a modified fly ash as an adsorbent for lead. WATER SA-PRETORIA 26(4):531–536
Woolard CD, Strong J, Erasmus CR (2002) Evaluation of the use of modified coal ash as a potential sorbent for organic waste streams. Appl Geochem 17(8):1159–1164. doi:10.1016/s0883-2927(02)00057-4
Wu D, Sui Y, He S, Wang X, Li C, Kong H (2008) Removal of trivalent chromium from aqueous solution by zeolite synthesized from coal fly ash. J Hazard Mater 155(3):415–423. doi:10.1016/j.jhazmat.2007.11.082
Xenidis A, Mylona E, Paspaliaris I (2002) Potential use of lignite fly ash for the control of acid generation from sulphidic wastes. Waste Manag 22(6):631–641. doi:10.1016/s0956-053x(01)00053-8
Yang EH, Yang Y, Li VC (2007) Use of high volumes of fly ash to improve ECC mechanical properties and material greenness. ACI Mater J 104(6):620–628
Yao Z, Xia M, Ye Y (2011) Dilithium dialuminium trisilicate crystalline phase prepared from coal fly ash. J Mater Eng Perform 1–5. doi:10.1007/s11665-011-9959-3
Yunusa IAM, Manoharan V, Odeh IOA, Shrestha S, Skilbeck CG, Eamus D (2011) Structural and hydrological alterations of soil due to addition of coal fly ash. J Soils Sediments 11(3):423–431. doi:10.1007/s11368-010-0312-5
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jayaranjan, M.L.D., van Hullebusch, E.D. & Annachhatre, A.P. Reuse options for coal fired power plant bottom ash and fly ash. Rev Environ Sci Biotechnol 13, 467–486 (2014). https://doi.org/10.1007/s11157-014-9336-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11157-014-9336-4