Abstract
Bone marrow contains resident cellular components that are not only involved in bone maintenance but also regulate hematopoiesis and immune responses. The immune system and bone interact with each other, coined osteoimmunology. Hashimoto’s thyroiditis (HT) is one of the most common chronic autoimmune diseases which is accompanied by lymphocytic infiltration. It shows elevating thyroid autoantibody levels at an early stage and progresses to thyroid dysfunction ultimately. Different effects exert on bone metabolism during different phases of HT. In this review, we summarized the mechanisms of the long-term effects of HT on bone and the relationship between thyroid autoimmunity and osteoimmunology. For patients with HT, the bone is affected not only by thyroid function and the value of TSH, but also by the setting of the autoimmune background. The autoimmune background implies a breakdown of the mechanisms that control self-reactive system, featuring abnormal immune activation and presence of autoantibodies. The etiology of thyroid autoimmunity and osteoimmunology is complex and involves a number of immune cells, cytokines and chemokines, which regulate the pathogenesis of HT and osteoporosis at the same time, and have potential to affect each other. In addition, vitamin D works as a potent immunomodulator to influence both thyroid immunity and osteoimmunology. We conclude that HT affects bone metabolism at least through endocrine and immune pathways.


Similar content being viewed by others
Abbreviations
- HT:
-
Hashimoto’s thyroiditis
- RANK:
-
receptor activator of nuclear factor-kappaB
- RANKL:
-
receptor activator of nuclear factor-kappaB ligand
- AIT:
-
autoimmune thyroiditis
- TH:
-
thyroid hormones
- TSH:
-
thyroid stimulating hormone
- T4:
-
thyroxine
- T3:
-
triiodothyronine
- OATP:
-
organic anion transporting polypeptide
- MCT:
-
monocarboxylate transporter
- SLC:
-
solute carriers
- DIO1:
-
type 1 iodothyronine deiodinase
- DIO2:
-
type 2 iodothyronine deiodinase
- DIO3:
-
type 3 iodothyronine deiodinase
- GWA:
-
genome-wide association
- HPT:
-
hypothalamic-pituitary-thyroid
- TR:
-
thyroid hormone nuclear receptor
- IGF-1:
-
insulin-like growth factor-1
- OPG:
-
osteoprotegerin
- LT4:
-
levothyroxine
- NK:
-
natural killer
- TgAb:
-
antibodies against thyroglobulin
- TPOAb:
-
antibodies against thyroid peroxidase
- CDC:
-
complement-dependent cytotoxicity
- ADCC:
-
antibody-dependent cell-mediated cytotoxicity
- FRAX:
-
Fracture Risk Assessment Tool
- 1,25(OH)2D:
-
1,25-dihydroxyvitamin D
- VDR:
-
vitamin D receptor
- IL:
-
interleukin
- ST2:
-
suppression of tumorigenicity 2 protein
- TNF-α:
-
tumor necrosis factor- alpha
- IFN-γ:
-
interferon-gamma
- L-SAT:
-
lymphocytic- spontaneous autoimmune thyroiditis
- TRAF6:
-
tumor necrosis factor receptor associated factor 6
- RUNX2:
-
runt-related transcription factor 2
- Se:
-
selenium
- SeNPs:
-
Se nanoparticles
- ALP:
-
alkaline phosphatase
References
Delitala AP, Scuteri A, Doria C. Thyroid Hormone Diseases and Osteoporosis. J Clin Med, 2020. 9(4).
Engblom C, et al., Osteoblasts remotely supply lung tumors with cancer-promoting SiglecF(high) neutrophils. Science, 2017. 358(6367).
Schett G, David JP. The multiple faces of autoimmune-mediated bone loss. Nat Rev Endocrinol. 2010;6(12):698–706.
Takegahara N, Kim H, Choi Y. RANKL biology Bone. 2022;159:116353.
Edner NM, et al. Targeting co-stimulatory molecules in autoimmune disease. Nat Rev Drug Discov. 2020;19(12):860–83.
Schett G, Takayanagi H. Editorial overview: Osteoimmunology Bone. 2022;162:116466.
Arron JR, Choi Y. Bone versus immune system. Nature. 2000;408(6812):535–6.
Lademann F, et al. Bone cell-specific deletion of thyroid hormone transporter Mct8 distinctly regulates bone volume in young versus adult male mice. Bone. 2022;159:116375.
Groeneweg S, et al., Thyroid Hormone Transporters. Endocr Rev, 2020. 41(2).
Williams AJ, et al. Iodothyronine deiodinase enzyme activities in bone. Bone. 2008;43(1):126–34.
Lademann F, et al. Lack of the thyroid hormone transporter Mct8 in osteoblast and osteoclast progenitors increases trabecular bone in male mice. Thyroid. 2020;30(2):329–42.
Abe S, et al. Monocarboxylate transporter 10 functions as a thyroid hormone transporter in chondrocytes. Endocrinology. 2012;153(8):4049–58.
Lademann F, et al., The thyroid hormone transporter MCT10 is a Novel Regulator of Trabecular Bone Mass and bone turnover in male mice. Endocrinology, 2022. 163(1).
Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010;31(2):139–70.
Lavado-Autric R, et al. Deiodinase activities in thyroids and tissues of iodine-deficient female rats. Endocrinology. 2013;154(1):529–36.
Gouveia CH, et al. Type 2 iodothyronine selenodeiodinase is expressed throughout the mouse skeleton and in the MC3T3-E1 mouse osteoblastic cell line during differentiation. Endocrinology. 2005;146(1):195–200.
Zaitune CR, et al. Abnormal thyroid hormone status differentially affects bone Mass Accrual and Bone Strength in C3H/HeJ mice: a mouse model of type I deiodinase Deficiency. Front Endocrinol (Lausanne). 2019;10:300.
Bassett JH, et al. Optimal bone strength and mineralization requires the type 2 iodothyronine deiodinase in osteoblasts. Proc Natl Acad Sci U S A. 2010;107(16):7604–9.
Dentice M, et al. The hedgehog-inducible ubiquitin ligase subunit WSB-1 modulates thyroid hormone activation and PTHrP secretion in the developing growth plate. Nat Cell Biol. 2005;7(7):698–705.
Waung JA, Bassett JH, Williams GR. Adult mice lacking the type 2 iodothyronine deiodinase have increased subchondral bone but normal articular cartilage. Thyroid. 2015;25(3):269–77.
Kang YE, et al. Type 2 deiodinase Thr92Ala polymorphism is associated with a reduction in bone mineral density: a community-based korean genome and epidemiology study. Clin Endocrinol (Oxf). 2020;93(3):238–47.
Zhang L, et al. Multistage genome-wide association meta-analyses identified two new loci for bone mineral density. Hum Mol Genet. 2014;23(7):1923–33.
Gogakos A, et al. THRA and DIO2 mutations are unlikely to be a common cause of increased bone mineral density in euthyroid post-menopausal women. Eur J Endocrinol. 2014;170(4):637–44.
Bassett JH, Williams GR, Critical role of the hypothalamic-pituitary-thyroid axis in bone Bone, 2008. 43(3): p. 418 – 26.
Abu EO, et al. The expression of thyroid hormone receptors in human bone. Bone. 1997;21(2):137–42.
Lademann F, et al. Disruption of BMP Signaling prevents Hyperthyroidism-Induced Bone loss in male mice. J Bone Miner Res. 2020;35(10):2058–69.
Siebler T, et al. Thyroid status affects number and localization of thyroid hormone receptor expressing mast cells in bone marrow. Bone. 2002;30(1):259–66.
Nicholls JJ, et al. The skeletal consequences of thyrotoxicosis. J Endocrinol. 2012;213(3):209–21.
Bassett JH, et al. Thyroid hormone excess rather than thyrotropin deficiency induces osteoporosis in hyperthyroidism. Mol Endocrinol. 2007;21(5):1095–107.
Monfoulet LE, et al. Thyroid hormone receptor β mediates thyroid hormone effects on bone remodeling and bone mass. J Bone Miner Res. 2011;26(9):2036–44.
Bassett JH, et al. Thyroid status during skeletal development determines adult bone structure and mineralization. Mol Endocrinol. 2007;21(8):1893–904.
Kaneshige M, et al. Mice with a targeted mutation in the thyroid hormone beta receptor gene exhibit impaired growth and resistance to thyroid hormone. Proc Natl Acad Sci U S A. 2000;97(24):13209–14.
O’Shea PJ, et al. Advanced bone formation in mice with a dominant-negative mutation in the thyroid hormone receptor β gene due to activation of Wnt/β-catenin protein signaling. J Biol Chem. 2012;287(21):17812–22.
Lindsey RC, Mohan S. Thyroid hormone acting via TRβ induces expression of browning genes in mouse bone marrow adipose tissue. Endocrine. 2017;56(1):109–20.
Bassett JH, Williams GR. Role of thyroid hormones in skeletal development and bone maintenance. Endocr Rev. 2016;37(2):135–87.
Stevens DA, et al. Thyroid hormone activates fibroblast growth factor receptor-1 in bone. Mol Endocrinol. 2003;17(9):1751–66.
Mundy GR, et al. Direct stimulation of bone resorption by thyroid hormones. J Clin Invest. 1976;58(3):529–34.
Allain TJ, et al. Tri-iodothyronine stimulates rat osteoclastic bone resorption by an indirect effect. J Endocrinol. 1992;133(3):327–31.
Siddiqi A, et al. Serum cytokines in thyrotoxicosis. J Clin Endocrinol Metab. 1999;84(2):435–9.
Lambrinoudaki I, et al. Thyroid function and autoimmunity are associated with the risk of vertebral fractures in postmenopausal women. J Bone Miner Metab. 2017;35(2):227–33.
Mazziotti G, et al. Serum TSH values and risk of vertebral fractures in euthyroid post-menopausal women with low bone mineral density. Bone. 2010;46(3):747–51.
Leader A, et al. Thyrotropin levels within the lower normal range are associated with an increased risk of hip fractures in euthyroid women, but not men, over the age of 65 years. J Clin Endocrinol Metab. 2014;99(8):2665–73.
Abrahamsen B, et al. Low serum thyrotropin level and duration of suppression as a predictor of major osteoporotic fractures-the OPENTHYRO register cohort. J Bone Miner Res. 2014;29(9):2040–50.
Soto-Pedre E, et al. Evidence of a causal relationship between serum thyroid-stimulating hormone and osteoporotic bone fractures. Eur Thyroid J. 2021;10(6):439–46.
Deng T, et al. Thyroid-stimulating hormone decreases the risk of osteoporosis by regulating osteoblast proliferation and differentiation. BMC Endocr Disord. 2021;21(1):49.
Konca Degertekin C, et al. RANKL/Osteoprotegerin system and bone turnover in Hashimoto Thyroiditis. Calcif Tissue Int. 2016;99(4):365–72.
Grimnes G, et al. The relationship between serum TSH and bone mineral density in men and postmenopausal women: the Tromsø study. Thyroid. 2008;18(11):1147–55.
Sun L, et al. Intermittent recombinant TSH injections prevent ovariectomy-induced bone loss. Proc Natl Acad Sci U S A. 2008;105(11):4289–94.
Baliram R, et al. Hyperthyroid-associated osteoporosis is exacerbated by the loss of TSH signaling. J Clin Invest. 2012;122(10):3737–41.
Abe E, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115(2):151–62.
Hase H, et al. TNFalpha mediates the skeletal effects of thyroid-stimulating hormone. Proc Natl Acad Sci U S A. 2006;103(34):12849–54.
Baliram R, et al. Thyroid-stimulating hormone induces a Wnt-dependent, feed-forward loop for osteoblastogenesis in embryonic stem cell cultures. Proc Natl Acad Sci U S A. 2011;108(39):16277–82.
Baliram R, et al. Thyroid and bone: macrophage-derived TSH-β splice variant increases murine osteoblastogenesis. Endocrinology. 2013;154(12):4919–26.
Boutin A, et al. β-Arrestin-1 mediates thyrotropin-enhanced osteoblast differentiation. Faseb j. 2014;28(8):3446–55.
Iddah MA, Macharia BN. Autoimmune thyroid disorders. ISRN Endocrinol. 2013;2013:509764.
Dunne C, De Luca F. Long-term Follow-Up of a child with autoimmune thyroiditis and recurrent hyperthyroidism in the absence of TSH receptor antibodies. Case Rep Endocrinol. 2014;2014:749576.
Shahbaz A, et al. Prolonged duration of hashitoxicosis in a patient with Hashimoto’s Thyroiditis: a Case Report and Review of Literature. Cureus. 2018;10(6):e2804.
Hennessey JV, et al. The Association between switching from Synthroid(®) and clinical outcomes: US evidence from a retrospective database analysis. Adv Ther. 2021;38(1):337–49.
Eriksen EF, Mosekilde L, Melsen F. Kinetics of trabecular bone resorption and formation in hypothyroidism: evidence for a positive balance per remodeling cycle. Bone. 1986;7(2):101–8.
Vestergaard P, Mosekilde L. Fractures in patients with hyperthyroidism and hypothyroidism: a nationwide follow-up study in 16,249 patients. Thyroid. 2002;12(5):411–9.
Bassett JH, et al. A lack of thyroid hormones rather than excess thyrotropin causes abnormal skeletal development in hypothyroidism. Mol Endocrinol. 2008;22(2):501–12.
Obling ML, et al. Restoration of euthyroidism in women with Hashimoto’s thyroiditis changes bone microarchitecture but not estimated bone strength. Endocrine. 2021;71(2):397–406.
Tuchendler D, Bolanowski M. Assessment of bone metabolism in premenopausal females with hyperthyroidism and hypothyroidism. Endokrynol Pol. 2013;64(1):40–4.
Blum MR, et al. Subclinical thyroid dysfunction and fracture risk: a meta-analysis. JAMA. 2015;313(20):2055–65.
Liang LB, et al. Changes of bone mineral density and bone metabolic marker in patients with subclinical hypothyroidism. Sichuan Da Xue Xue Bao Yi Xue Ban. 2014;45(1):83. 66 – 9,, , ( : p.
Polovina S, et al. Frax score calculations in postmenopausal women with subclinical hypothyroidism. Horm (Athens). 2013;12(3):439–48.
Nagata M, et al. Subclinical hypothyroidism is related to lower heel QUS in postmenopausal women. Endocr J. 2007;54(4):625–30.
Mosekilde L, Eriksen EF, Charles P. Effects of thyroid hormones on bone and mineral metabolism. Endocrinol Metab Clin North Am. 1990;19(1):35–63.
Reverter JL, et al. Lack of deleterious effect on bone mineral density of long-term thyroxine suppressive therapy for differentiated thyroid carcinoma. Endocr Relat Cancer. 2005;12(4):973–81.
Appetecchia M. Effects on bone mineral density by treatment of benign nodular goiter with mildly suppressive doses of L-thyroxine in a cohort women study. Horm Res. 2005;64(6):293–8.
Mendonça M, de Barros G, et al. Bone mineral density and bone microarchitecture after long-term suppressive levothyroxine treatment of differentiated thyroid carcinoma in young adult patients. J Bone Miner Metab. 2016;34(4):417–21.
Yoon BH, et al. Influence of thyroid-stimulating hormone suppression therapy on bone Mineral density in patients with differentiated thyroid Cancer: a Meta-analysis. J Bone Metab. 2019;26(1):51–60.
Ku EJ, et al. Effect of TSH suppression therapy on bone Mineral density in differentiated thyroid Cancer: a systematic review and Meta-analysis. J Clin Endocrinol Metab. 2021;106(12):3655–67.
Davidson A, Diamond B. Autoimmune diseases. N Engl J Med. 2001;345(5):340–50.
Ganesh BB, et al. Role of cytokines in the pathogenesis and suppression of thyroid autoimmunity. J Interferon Cytokine Res. 2011;31(10):721–31.
Crane IJ, Forrester JV. Th1 and Th2 lymphocytes in autoimmune disease. Crit Rev Immunol. 2005;25(2):75–102.
Zake T, et al. Upregulated tissue expression of T helper (th) 17 pathogenic interleukin (IL)-23 and IL-1β in Hashimoto’s thyroiditis but not in Graves’ disease. Endocr J. 2019;66(5):423–30.
Huifang S. Study on the correlation between Hashimoto’s thyroiditis and bone mineral density in postmenopausal women. Qingdao University; 2021.
Polovina SP, et al. The impact of thyroid autoimmunity (TPOAb) on bone density and fracture risk in postmenopausal women. Horm (Athens). 2017;16(1):54–61.
Siris ES, Baim S, Nattiv A. Primary care use of FRAX: absolute fracture risk assessment in postmenopausal women and older men. Postgrad Med. 2010;122(1):82–90.
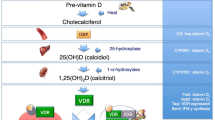
Koehler VF, Filmann N, Mann WA. Vitamin D status and thyroid autoantibodies in Autoimmune Thyroiditis. Horm Metab Res. 2019;51(12):792–7.
van de Peppel J, van Leeuwen JP. Vitamin D and gene networks in human osteoblasts. Front Physiol. 2014;5:137.
Takeda S, et al. Stimulation of osteoclast formation by 1,25-dihydroxyvitamin D requires its binding to vitamin D receptor (VDR) in osteoblastic cells: studies using VDR knockout mice. Endocrinology. 1999;140(2):1005–8.
Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94(1):26–34.
Hossein-nezhad A, Spira A, Holick MF. Influence of vitamin D status and vitamin D3 supplementation on genome wide expression of white blood cells: a randomized double-blind clinical trial. PLoS ONE. 2013;8(3):e58725.
Lechner J, Aschoff J, Rudi T. The vitamin D receptor and the etiology of RANTES/CCL-expressive fatty-degenerative osteolysis of the jawbone: an interface between osteoimmunology and bone metabolism. Int J Gen Med. 2018;11:155–66.
Chahardoli R, et al., Can Supplementation with Vitamin D Modify Thyroid Autoantibodies (Anti-TPO Ab, Anti-Tg Ab) and Thyroid Profile (T3, T4, TSH) in Hashimoto’s Thyroiditis? A Double Blind, Randomized Clinical Trial Horm Metab Res, 2019. 51(5): p. 296–301.
Fang F, et al. Vitamin D deficiency is associated with thyroid autoimmunity: results from an epidemiological survey in Tianjin, China. Endocrine. 2021;73(2):447–54.
Khozam SA, et al. Association between vitamin D Deficiency and Autoimmune thyroid disorder: a systematic review. Cureus. 2022;14(6):e25869.
Jiang H, et al. Effects of vitamin D treatment on thyroid function and autoimmunity markers in patients with Hashimoto’s thyroiditis-A meta-analysis of randomized controlled trials. J Clin Pharm Ther. 2022;47(6):767–75.
Nodehi M, et al. Effects of vitamin D supplements on frequency of CD4(+) T-cell subsets in women with Hashimoto’s thyroiditis: a double-blind placebo-controlled study. Eur J Clin Nutr. 2019;73(9):1236–43.
Altieri B, et al. Does vitamin D play a role in autoimmune endocrine disorders? A proof of concept. Rev Endocr Metab Disord. 2017;18(3):335–46.
Sarmiento-Ramón MP, et al. Characterization of serum vitamin D levels in pediatric patients with chronic lymphocytic thyroiditis. Bol Med Hosp Infant Mex. 2022;79(3):161–9.
Kaan Demircioglu M, et al. Is vitamin D Deficiency Associated with chronic lymphocytic thyroiditis? Sisli Etfal Hastan Tip Bul. 2021;55(4):510–5.
Maciejewski A, et al. Vitamin D receptor gene polymorphisms and autoimmune thyroiditis: are they Associated with Disease occurrence and its features? Biomed Res Int. 2019;2019:8197580.
Behera KK, et al. Effect of vitamin D supplementation on thyroid autoimmunity among subjects of autoimmune thyroid disease in a Coastal Province of India: a Randomized Open-label trial. Niger Med J. 2020;61(5):237–40.
Azam, Amini, et al. The effect of vitamin D replacement on Musculoskeletal Pain in hypothyroid patients with vitamin D Deficiency. Int J Sci Eng Res. 2017;8(5):640–2.
Wang H, et al., Associations between dynamic vitamin D level and thyroid function during pregnancy. Nutrients, 2022. 14(18).
Chen Y, et al. Vitamin D categories and postpartum thyroid function in women with hypothyroidism. Front Nutr. 2022;9:953745.
Xu Z, et al. SMURF2 regulates bone homeostasis by disrupting SMAD3 interaction with vitamin D receptor in osteoblasts. Nat Commun. 2017;8:14570.
Hofbauer LC, et al. Detection and characterization of RANK ligand and osteoprotegerin in the thyroid gland. J Cell Biochem. 2002;86(4):642–50.
Nagasaki T, et al. Increased levels of serum osteoprotegerin in hypothyroid patients and its normalization with restoration of normal thyroid function. Eur J Endocrinol. 2005;152(3):347–53.
Guang-da X, et al. Changes in plasma concentrations of osteoprotegerin before and after levothyroxine replacement therapy in hypothyroid patients. J Clin Endocrinol Metab. 2005;90(10):5765–8.
Giusti M, et al. Serum osteoprotegerin and soluble receptor activator of nuclear factor kappaB ligand levels in patients with a history of differentiated thyroid carcinoma: a case-controlled cohort study. Metabolism. 2007;56(5):699–707.
Giusti M, et al. Recombinant human thyroid stimulating hormone does not acutely change serum osteoprotegerin and soluble receptor activator of nuclear factor-kappabeta ligand in patients under evaluation for differentiated thyroid carcinoma. Horm (Athens). 2007;6(4):304–13.
Ginaldi L, De Martinis M. Osteoimmunology and Beyond. Curr Med Chem. 2016;23(33):3754–74.
Brincat SD, et al. The role of cytokines in postmenopausal osteoporosis. Minerva Ginecol. 2014;66(4):391–407.
Chen B, Li HZ. Association of IL-6 174G/C (rs1800795) and 572 C/G (rs1800796) polymorphisms with risk of osteoporosis: a meta-analysis. BMC Musculoskelet Disord. 2020;21(1):330.
Poli V, et al. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. Embo j. 1994;13(5):1189–96.
Sims NA, et al. Glycoprotein 130 regulates bone turnover and bone size by distinct downstream signaling pathways. J Clin Invest. 2004;113(3):379–89.
Liu XH, et al. Interactive effect of interleukin-6 and prostaglandin E2 on osteoclastogenesis via the OPG/RANKL/RANK system. Ann N Y Acad Sci. 2006;1068:225–33.
Honda K. Interleukin-6 and soluble interleukin-6 receptor suppress osteoclastic differentiation by inducing PGE(2) production in chondrocytes. J Oral Sci. 2011;53(1):87–96.
Lakatos P, et al. Serum interleukin-6 and bone metabolism in patients with thyroid function disorders. J Clin Endocrinol Metab. 1997;82(1):78–81.
Gerenova J, Manolova I, Stanilova S. SERUM LEVELS OF INTERLEUKIN – 23 AND INTERLEUKIN – 17 IN HASHIMOTO’S THYROIDITIS. Acta Endocrinol (Buchar). 2019;-5(1):74–9.
Li Q, et al. The pathogenesis of thyroid autoimmune diseases: new T lymphocytes - cytokines circuits beyond the Th1-Th2 paradigm. J Cell Physiol. 2019;234(3):2204–16.
Pan Y, et al. A chinese patent Medicine JiaYanKangTai alleviates inflammatory lesions of experimental autoimmune thyroiditis by regulating Interleukin-17 signaling. Front Endocrinol (Lausanne). 2021;12:794568.
Liu Y, et al. Elevated MicroRNA-326 levels regulate the IL-23/IL-23R/Th17 cell Axis in Hashimoto’s Thyroiditis by targeting a disintegrin and metalloprotease 17. Thyroid. 2020;30(9):1327–37.
Figueroa-Vega N, et al. Increased circulating pro-inflammatory cytokines and Th17 lymphocytes in Hashimoto’s thyroiditis. J Clin Endocrinol Metab. 2010;95(2):953–62.
Bhadricha H, et al. Increased frequency of Th17 cells and IL-17 levels are associated with low bone mineral density in postmenopausal women. Sci Rep. 2021;11(1):16155.
Ikeuchi T, Moutsopoulos NM. Osteoimmunology in periodontitis; a paradigm for Th17/IL-17 inflammatory bone loss. Bone, 2022: p. 116500.
Kim YG, et al. IL-17 inhibits osteoblast differentiation and bone regeneration in rat. Arch Oral Biol. 2014;59(9):897–905.
Zhang JR, et al. Different Modulatory Effects of IL-17, IL-22, and IL-23 on osteoblast differentiation. Mediators Inflamm. 2017;2017:5950395.
Kim HJ, et al. IL-17 promotes osteoblast differentiation, bone regeneration, and remodeling in mice. Biochem Biophys Res Commun. 2020;524(4):1044–50.
Uluçkan Ö, et al. Chronic skin inflammation leads to bone loss by IL-17-mediated inhibition of wnt signaling in osteoblasts. Sci Transl Med. 2016;8(330):330ra37.
Gaffen SL, et al. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14(9):585–600.
Ruggeri RM, et al. Serum interleukin-23 (IL-23) is increased in Hashimoto’s thyroiditis. Endocr J. 2014;61(4):359–63.
Shukla P, Mansoori MN, Singh D. Efficacy of anti-IL-23 monotherapy versus combination therapy with anti-IL-17 in estrogen deficiency induced bone loss conditions. Bone. 2018;110:84–95.
Adamopoulos IE, et al. IL-23 is critical for induction of arthritis, osteoclast formation, and maintenance of bone mass. J Immunol. 2011;187(2):951–9.
Ju JH, et al. IL-23 induces receptor activator of NF-kappaB ligand expression on CD4 + T cells and promotes osteoclastogenesis in an autoimmune arthritis model. J Immunol. 2008;181(2):1507–18.
Kang YK, Zhang MC. IL-23 promotes osteoclastogenesis in osteoblast-osteoclast co-culture system. Genet Mol Res. 2014;13(2):4673–9.
Razawy W, et al. IL-23 receptor deficiency results in lower bone mass via indirect regulation of bone formation. Sci Rep. 2021;11(1):10244.
Kamiya S, et al. Effects of IL-23 and IL-27 on osteoblasts and osteoclasts: inhibitory effects on osteoclast differentiation. J Bone Miner Metab. 2007;25(5):277–85.
Wang X, et al. Dysregulated interleukin – 33/ST2 pathway perpetuates chronic inflammation in Hashimoto’s Thyroiditis. Endocr Metab Immune Disord Drug Targets. 2019;19(7):1012–21.
Mun SH, et al. Interleukin-33 stimulates formation of functional osteoclasts from human CD14(+) monocytes. Cell Mol Life Sci. 2010;67(22):3883–92.
Malcolm J, et al. IL-33 exacerbates Periodontal Disease through induction of RANKL. J Dent Res. 2015;94(7):968–75.
Zaiss MM, et al. IL-33 shifts the balance from osteoclast to alternatively activated macrophage differentiation and protects from TNF-alpha-mediated bone loss. J Immunol. 2011;186(11):6097–105.
Kaneko N, et al. The role of interleukin-1 in general pathology. Inflamm Regen. 2019;39:12.
Mine M, et al. Interleukin-1 stimulates thyroid cell growth and increases the concentration of the c-myc proto-oncogene mRNA in thyroid follicular cells in culture. Endocrinology. 1987;120(3):1212–4.
Enomoto T, et al. Prolonged effects of recombinant human interleukin-1 alpha on mouse thyroid function. Endocrinology. 1990;127(5):2322–7.
Nilsson M, et al. Cytokines and thyroid epithelial integrity: interleukin-1alpha induces dissociation of the junctional complex and paracellular leakage in filter-cultured human thyrocytes. J Clin Endocrinol Metab. 1998;83(3):945–52.
Yamashita S, et al. Interleukin-1 inhibits thyrotrophin-induced human thyroglobulin gene expression. J Endocrinol. 1989;122(1):177–83.
Ashizawa K, et al. Inhibition of human thyroid peroxidase gene expression by interleukin 1. Acta Endocrinol (Copenh). 1989;121(4):465–9.
Zhao R, Zhou H, Su SB. A critical role for interleukin-1β in the progression of autoimmune diseases. Int Immunopharmacol. 2013;17(3):658–69.
Sun L, et al. Elevated interleukin-1β in peripheral blood mononuclear cells contributes to the pathogenesis of autoimmune thyroid diseases, especially of Hashimoto thyroiditis. Endocr Res. 2016;41(3):185–92.
Levescot A, et al., IL-1β-driven osteoclastogenic Tregs accelerate bone erosion in arthritis. J Clin Invest, 2021. 131(18).
Hengartner NE, et al. IL-1β inhibits human osteoblast migration. Mol Med. 2013;19(1):36–42.
Ebe Y, et al. Effect of interleukin-1β on bone morphogenetic protein-9-induced osteoblastic differentiation of human periodontal ligament fibroblasts. Eur J Oral Sci. 2021;129(4):e12792.
Huang J, Chen L. IL-1β inhibits osteogenesis of human bone marrow-derived mesenchymal stem cells by activating FoxD3/microRNA-496 to repress wnt signaling. Genesis, 2017. 55(7).
Ye W, et al. IL-1β-Treated bone marrow mesenchymal stem cells enhances osteogenetic potential via NF-κB pathway. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2017;25(3):890–5.
Hong SH, Braley-Mullen H. Follicular B cells in thyroids of mice with spontaneous autoimmune thyroiditis contribute to disease pathogenesis and are targets of anti-CD20 antibody therapy. J Immunol. 2014;192(3):897–905.
Mariotti S, et al. Recent advances in the understanding of humoral and cellular mechanisms implicated in thyroid autoimmune disorders. Clin Immunol Immunopathol. 1989;50(1 Pt 2):S73–84.
Yongshuang Xie WQ. Researching on the relation between autoimmune thyroid disesae and tumor necrosis factors-alpha/interleukin-6 ratio. J Practical Med Techniques. 2009;16:425–6.
Braley-Mullen H, Yu S. NOD.H-2h4 mice: an important and underutilized animal model of autoimmune thyroiditis and Sjogren’s syndrome. Adv Immunol. 2015;126:1–43.
Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118(11):3537–45.
Boehm U, et al. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–95.
Yu S, Sharp GC, Braley-Mullen H. Dual roles for IFN-gamma, but not for IL-4, in spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Immunol. 2002;169(7):3999–4007.
Yu S, Sharp GC, Braley-Mullen H. Thyrocytes responding to IFN-gamma are essential for development of lymphocytic spontaneous autoimmune thyroiditis and inhibition of thyrocyte hyperplasia. J Immunol. 2006;176(2):1259–65.
Zhang X, Zhu L, Sun L, IFN-y mediates thyroid injury by upregulating Fas expression in Hashimoto thyroiditis Acta Universitatis Medicinalis Anhui, 2017. 52(06): p. 806–809.
Gao Y, et al. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J Clin Invest. 2007;117(1):122–32.
Takayanagi H, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;408(6812):600–5.
Maruhashi T, et al. DCIR maintains bone homeostasis by regulating IFN-γ production in T cells. J Immunol. 2015;194(12):5681–91.
Hu Y, et al. Effect of selenium on thyroid autoimmunity and regulatory T cells in patients with Hashimoto’s thyroiditis: a prospective randomized-controlled trial. Clin Transl Sci. 2021;14(4):1390–402.
Kryczyk-Kozioł J, et al., Assessment of the Effect of Selenium supplementation on production of selected cytokines in women with Hashimoto’s Thyroiditis. Nutrients, 2022. 14(14).
Poleboina S, et al. Selenium nanoparticles stimulate osteoblast differentiation via BMP-2/MAPKs/β-catenin pathway in diabetic osteoporosis. Nanomed (Lond). 2022;17(9):607–25.
Wei M, et al. Manganese, iron, copper, and selenium co-exposure and osteoporosis risk in chinese adults. J Trace Elem Med Biol. 2022;72:126989.
Beukhof CM, et al. Selenium Status is positively Associated with Bone Mineral density in healthy aging european men. PLoS ONE. 2016;11(4):e0152748.
Hoeg A, et al. Bone turnover and bone mineral density are independently related to selenium status in healthy euthyroid postmenopausal women. J Clin Endocrinol Metab. 2012;97(11):4061–70.
Wu CC, et al. Selenium status is independently related to bone mineral density, FRAX score, and bone fracture history: NHANES, 2013 to 2014. Bone. 2021;143:115631.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Statements and declarations
Funding.
This work was supported by grants from the National Natural Science Foundation of China [No. 82273294]; the Science and Technology Department of Sichuan Province (2022YFS0136); the Chengdu Bureau of Science and Technology (2022-YF05-01316-SN); the Sichuan University [No. 2018SCUH0093]; and the 1.3.5 project for discipline of excellence, West China Hospital, Sichuan University [No. 2020HXFH008, No. ZYJC18003].
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, J., Huang, H. & Yu, X. How does Hashimoto’s thyroiditis affect bone metabolism?. Rev Endocr Metab Disord 24, 191–205 (2023). https://doi.org/10.1007/s11154-022-09778-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-022-09778-x