Abstract
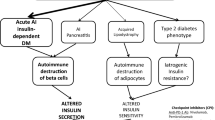
Immune Check-Point Inhibitors (CPIs) have improved long-term patients’ outcomes in several advanced cancers. Diabetes mellitus induced by CPIs (CPI-DM) is considered the second most frequent endocrine CPIs’ side effects with a variable prevalence up to 2%. The aim of our study was to identify CPI-DM characteristics and differences from the classical form of diabetes. Therefore, we conducted a structured Pubmed® search collecting publications dated from January 2015 to December 2019. A total of 642 citations were identified and 121 publications met our study criteria. We analyzed 200 case reports, including our 3 cases under publication. The majority of CPI-DM occurred with anti-Programmed cell Death-1 in monotherapy or in combination, although few cases with Programmed cell Death Ligand-1 and Cytotoxic T Lymphocyte Antigen 4 were reported. Generally, CPI-DM arose early (an average of 9 weeks after CPIs starting), but also after the end of CPIs treatment. In all patients, CPI-DM has an acute onset and in 67.5% of cases diabetic ketoacidosis occurs. C-peptide levels were usually and permanently compromised, requiring lifelong insulin therapy. Moreover, autoimmunity and genetic profile was not always helpful. In particular, anti-glutamic acid decarboxylase (anti-GAD) antibodies and Human Leukocyte Antigen (HLA) DR4 were present in only 43.0% and 51.3% of cases respectively. In 51.0% of subjects a mild exocrine impairment coexisted. In short, though CPI-DM has similarities to type 1 diabetes mellitus, it represents a new, largely unknown, clinical entity. In addition, as CPI-DM is a relative frequent side-effect under CPI, a close monitoring of the glucose levels and early signs and symptoms of diabetes in patients affected by neoplasm is recommended.





Similar content being viewed by others
Abbreviations
- AGL-CPI:
-
Autoimmune generalized lipodystrophy induced by check-point inhibitors
- Anti-GAD:
-
Anti-glutamic acid decarboxylase antibodies
- Anti-IA2:
-
Islet antigen-2 antibodies
- Anti-PD1:
-
Anti-programmed cell Death 1
- CPI-DM:
-
Diabetes Mellitus induced by check-point inhibitors
- CPI-FD:
-
Fulminant diabetes induced by check-point inhibitors
- CPIs:
-
Check-point inhibitors
- CR:
-
Complete response
- CTLA-4:
-
Cytotoxic T Lymphocyte antigen 4
- DKA:
-
Diabetic ketoacidosis.
- DM:
-
Diabetes mellitus
- FD:
-
Fulminant diabetes
- HLA:
-
Human leukocyte antigen
- IAA:
-
Insulin auto-antibodies
- IrAEs:
-
Immune-related adverse events
- MDI:
-
Multi-dose insulin
- PD:
-
Progression disease
- PD1:
-
Programmed cell Death 1
- PD-L1:
-
Programmed cell Death 1 Ligand 1
- PD-L2:
-
Programmed cell Death 1 Ligand 2
- PR:
-
Partial response
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analysis methods
- SD:
-
Stable disease
- T1DM:
-
Type 1 Diabetes Mellitus
- T2DM:
-
Type 2 Diabetes Mellitus
References
Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366(26):2517–9.
Cukier P, Santini FC, Scaranti M, Hoff AO. Endocrine side effects of cancer immunotherapy. Endocr Relat Cancer. 2017;24(12):T331–47.
Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, Tolaney SM. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):173–82.
Kotwal A, Haddox C, Block M, Kudva YC. Immune checkpoint inhibitors: an emerging cause of insulin-dependent diabetes. BMJ Open Diabetes Res Care. 2019;7(1):e000591.
Tsang VHM, McGrath RT, Clifton-Bligh RJ, Scolyer RA, Jakrot V, Guminski AD, et al. Checkpoint inhibitor–associated autoimmune diabetes is distinct from type 1 diabetes. J Clin Endocrinol Metab. 2019;104(11):5499–506.
Lu J, Yang J, Liang Y, Meng H, Zhao J, Zhang X. Incidence of immune checkpoint inhibitor-associated diabetes: a meta-analysis of randomized controlled studies. Front Pharmacol. 2019;10:1453.
Clotman K, Janssens K, Specenier P, Weets I, De Block CEM. Programmed cell death-1 inhibitor–induced type 1 diabetes mellitus. J Clin Endocrinol Metab. 2018;103(9):3144–54.
Tan MH, Iyengar R, Mizokami-Stout K, Yentz S, MacEachern MP, Shen LY, et al. Spectrum of immune checkpoint inhibitors-induced Endocrinopathies in Cancer patients: a scoping review of case reports. Clin Diabetes Endocrinol. 2019;5(1):1.
Farina KA, Kane MP. Programmed cell death-1 monoclonal antibody therapy and type 1 diabetes mellitus: a review of the literature. J Pharm Pract. 2019:089719001985092.
Marchand L, Disse E, Dalle S, Reffet S, Vouillarmet J, Fabien N, Thivolet C, Cugnet-Anceau C. The multifaceted nature of diabetes mellitus induced by checkpoint inhibitors. Acta Diabetol. 2019;56(12):1239–45.
de Filette JMK, Pen JJ, Decoster L, Vissers T, Bravenboer B, Van der Auwera BJ, et al. Immune checkpoint inhibitors and type 1 diabetes mellitus: a case report and systematic review. Eur J Endocrinol. 2019;181(3):363–74.
Gaudy C, Clévy C, Monestier S, Dubois N, Préau Y, Mallet S, Richard MA, Grob JJ, Valéro R, Béliard S. Anti-PD1 pembrolizumab can induce exceptional fulminant type 1 diabetes. Diabetes Care. 2015;38(11):e182–3.
Marchand L, Thivolet A, Dalle S, Chikh K, Reffet S, Vouillarmet J, Fabien N, Cugnet-Anceau C, Thivolet C. Diabetes mellitus induced by PD-1 and PD-L1 inhibitors: description of pancreatic endocrine and exocrine phenotype. Acta Diabetol. 2019;56(4):441–8.
Imagawa A, Hanafusa T, Miyagawa J, Matsuzawa Y. A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes-related antibodies. Osaka IDDM Study Group. N Engl J Med. 2000;342(5):301–7.
Kawasaki E, Eguchi K. Is type 1 diabetes in the Japanese population the same as among Caucasians? Ann N Y Acad Sci. 2004;1037(1):96–103.
Sue M, Yoshihara A, Otani T, Tsuchida Y, Higa M, Hiroi N. Characteristics of fulminant type 1 diabetes mellitus. Med Sci Monit. 2008;14(10):CS97–101.
Kawabata Y, Ikegami H, Awata T, Imagawa A, Maruyama T, Kawasaki E, et al. Committee on type 1 diabetes, Japan diabetes society. Differential association of HLA with three subtypes of type 1 diabetes: fulminant, slowly progressive and acute-onset. Diabetologia. 2009;52(12):2513–21.
Moreau C, Drui D, Arnault-Ouary G, Charbonnel B, Chaillous L, Cariou B. Fulminant type 1 diabetes in Caucasians: a report of three cases. Diabetes Metab. 2008;34(5):529–32.
Hosokawa Y, Hanafusa T, Imagawa A. Pathogenesis of fulminant type 1 diabetes: genes, viruses and the immune mechanism, and usefulness of patient-derived induced pluripotent stem cells for future research. J Diabetes Investig. 2019;10(5):1158–64.
Wright LAC, Ramon RV, Batacchi Z, Hirsch IB. Progression to insulin dependence post-treatment with immune checkpoint inhibitors in pre-existing type 2 diabetes. AACE Clin Case Rep. 2017;3(2):e153–7.
Wright JJ, Salem JE, Johnson DB, Lebrun-Vignes B, Stamatouli A, Thomas JW, Herold KC, Moslehi J, Powers AC. Increased reporting of immune checkpoint inhibitor–associated diabetes. Diabetes Care. 2018;41(12):e150–1.
Ansari MJI, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, Yamazaki T, Azuma M, Iwai H, Khoury SJ, Auchincloss H Jr, Sayegh MH. The programmed Death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198(1):63–9.
Martinov T., Spanier JA, Pauken KE, Fife BT (2016) PD-1 pathway-mediated regulation of islet-specific CD4+ T cell subsets in autoimmune diabetes. Immunoendocrinology 3, pii: e1164.
Tsiogka A, Jansky GL, Bauer JW, Koelblinger P. Fulminant type 1 diabetes after adjuvant Ipilimumab therapy in cutaneous melanoma. Melanoma Res. 2017;27(5):524–5.
Long SA, Rieck M, Sanda S, Bollyky JB, Samuels PL, Goland R, Ahmann A, Rabinovitch A, Aggarwal S, Phippard D, Turka LA, Ehlers MR, Bianchine PJ, Boyle KD, Adah SA, Bluestone JA, Buckner JH, Greenbaum CJ, for Diabetes TrialNet and the Immune Tolerance Network. Diabetes TrialNet and the immune tolerance network. Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs β-cell function. Diabetes. 2012;61(9):2340–8.
Nakamura K, Kawasaki E, Imagawa A, Awata T, Ikegami H, Uchigata Y, Kobayashi T, Shimada A, Nakanishi K, Makino H, Maruyama T, Hanafusa T. Research Committee on Type 1 diabetes of the Japan Diabetes Society. Type 1 diabetes and interferon therapy: a nationwide survey in Japan. Diabetes Care. 2011;34(9):2084–9.
Rodriguez-Calvo T, Ekwall O, Amirian N, Zapardiel-Gonzalo J, von Herrath MG. Increased immune cell infiltration of the exocrine pancreas: a possible contribution to the pathogenesis of type 1 diabetes. Diabetes. 2014;63(11):3880–90.
Perdigoto AL, Quandt Z, Anderson M, Herold KC. Checkpoint inhibitor-induced insulin-dependent diabetes: an emerging syndrome. Lancet Diabetes Endocrinol. 2019;7(6):421–3.
Akturk HK, Kahramangil D, Sarwal A, Hoffecker L, Murad MH, Michels AW. Immune checkpoint inhibitor-induced type 1 diabetes: a systematic review and meta-analysis. Diabet Med. 2019;36(9):1075–81.
Pihoker C, Gilliam LK, Hampe CS, Lernmark Å. Autoantibodies in diabetes. 2005;54(Suppl 2):S52–61.
Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA, Gettinger S, Sznol M, Young A, Rushakoff R, Lee J, Bluestone JA, Anderson M, Herold KC. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes. 2018;67(8):1471–80.
Matsuura N, Koh G, Konishi C, Minamino S, Takahara Y, Harada H, Kodama K, Emoto M. Fulminant onset of insulin-dependent diabetes with positive anti-GAD antibody titers during treatment with Nivolumab in a patient with NSCLC. Cancer Immunol Immunother. 2018;67(9):1417–24.
Cheema A, Makadia B, Karwadia T, Bajwa R, Hossain M. Autoimmune diabetes associated with Pembrolizumab: a review of published case reports. World J Oncol. 2018;9(1):1–4.
Noble JA, Valdes AM. Genetics of the HLA region in the prediction of type 1 diabetes. Curr Diab Rep. 2011;11(6):533–42.
Kapke J, Shaheen Z, Kilari D, Knudson P, Wong S. Immune checkpoint inhibitor-associated type 1 diabetes mellitus: case series, review of the Literature, and optimal management. Case Rep Oncol. 2017;10(3):897–909.
Miyoshi Y, Ogawa O, Oyama Y. Nivolumab, an anti-programmed cell Death-1 antibody, induces fulminant type 1 diabetes. Tohoku J Exp Med. 2016;239(2):155–8.
Fukui A, Sugiyama K, Yamada T. A case of nivolumab-induced fulminant type 1 diabetes with steroids and glucagon-like peptide 1 administration during the early onset. J Clin Case Rep. 2016;6:11.
Okamoto M, Okamoto M, Gotoh K, Masaki T, Ozeki Y, Ando H, Anai M, Sato A, Yoshida Y, Ueda S, Kakuma T, Shibata H. Fulminant type 1 diabetes mellitus with anti-programmed cell Death-1 therapy. J. Diabetes Investig. 2016;7(6):915–8.
Matsumura K, Nagasawa K, Oshima Y, Kikuno S, Hayashi K, Nishimura A, Okubo M, Uruga H, Kishi K, Kobayashi T, Mori Y. Aggravation of diabetes, and incompletely deficient insulin secretion in a case with type 1 diabetes-resistant human leukocyte antigen DRB1*15:02 treated with Nivolumab. J. Diabetes Investig. 2018;9(2):438–41.
Yamamoto N, Tsurutani Y, Katsuragawa S, Kubo H, Sunouchi T, Hirose R, Hoshino Y, Ichikawa M, Takiguchi T, Yukawa H, Arioka H, Saitou J, Nishikawa T. A patient with Nivolumab-related fulminant type 1 diabetes mellitus whose serum C-peptide level was preserved at the initial detection of hyperglycemia. Intern Med. 2019;58(19):2825–30.
Teramoto Y, Nakamura Y, Asami Y, Imamura T, Takahira S, Nemoto M, Sakai G, Shimada A, Noda M, Yamamoto A. Case of type 1 diabetes associated with less-dose Nivolumab therapy in a melanoma patient. J Dermatol. 2017;44(5):605–6.
Greenbaum CJ, Beam CA, Boulware D, Gitelman SE, Gottlieb PA, Herold KC, Lachin JM, McGee P, Palmer JP, Pescovitz MD, Krause-Steinrauf H, Skyler JS, Sosenko JM. On behalf of the type 1 diabetes TrialNet study group. Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite type 1 diabetes TrialNet data. Diabetes. 2012;61(8):2066–73.
Hansen E, Sahasrabudhe D, Sievert L. A case report of insulin-dependent diabetes as immune-related toxicity of Pembrolizumab: presentation. Manag Outcome Cancer Immunol Immunother. 2016;65(6):765–7.
Trinh B, Donath MY, Läubli H. Successful treatment of immune checkpoint inhibitor–induced diabetes with infliximab. Diabetes Care. 2019;42(9):e153–4.
Qiao YC, Chen YL, Pan YH, Tian F, Xu Y, Zhang XX, Zhao HL. The change of serum tumor necrosis factor alpha in patients with type 1 diabetes mellitus: a systematic review and meta-analysis. PLoS One. 2017;12(4):e0176157.
Mastrandrea L, Yu J, Behrens T, Buchlis J, Albini C, Fourtner S, Quattrin T. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled. Double-Blind Study Diabetes Care. 2009;32(7):1244–9.
Chae YK, Chiec L, Mohindra N, Gentzler R, Patel J, Giles F. A case of Pembrolizumab-induced Type-1 diabetes mellitus and discussion of immune checkpoint inhibitor-induced type 1 diabetes. Cancer Immunol Immunother. 2017;66(1):25–32.
Smith-Cohn MA, Gill D, Voorhies BN, Agarwal N, Garrido-Laguna I. Case report: Pembrolizumab-induced type 1 diabetes in a patient with metastatic Cholangiocarcinoma. Immunotherapy. 2017;9(10):797–804.
Ho WJ, Rooper L, Sagorsky S, Kang H. A robust response to combination immune checkpoint inhibitor therapy in HPV-related small cell cancer: a case report. J Immunother Cancer. 2018;6(1):33.
Villarreal J, Townes D, Vrablik M, Ro K. A case of drug-induced severe Endocrinopathies: what providers in the emergency department need to know. Adv Emerg Nurs J. 2018;40(1):16–20.
Sakaguchi C, Ashida K, Yano S, Ohe K, Wada N, Hasuzawa N, et al. A case of Nivolumab-induced acute-onset type 1 diabetes mellitus in melanoma. Curr Oncol. 2019;26(1):e115–8.
Williams AJK, Thrower SL, Sequeiros IM, Ward A, Bickerton AS, Triay JM, Callaway MP, Dayan CM. Pancreatic volume is reduced in adult patients with recently diagnosed type 1 diabetes. J Clin Endocrinol Metab. 2012;97(11):E2109–13.
Campbell-Thompson M, Wasserfall C, Montgomery EL, Atkinson MA, Kaddis JS. Pancreas organ weight in individuals with disease-associated autoantibodies at risk for type 1 diabetes. JAMA. 2012;308(22):2337–9.
Sayama K, Imagawa A, Okita K, Uno S, Moriwaki M, Kozawa J, Iwahashi H, Yamagata K, Tamura S, Matsuzawa Y, Hanafusa T, Miyagawa J, Shimomura I. Pancreatic beta and alpha cells are both decreased in patients with fulminant type 1 diabetes: a morphometrical assessment. Diabetologia. 2005;48(8):1560–4.
Sherr J, Tsalikian E, Fox L, Buckingham B, Weinzimer S, Tamborlane WV, White NH, Arbelaez AM, Kollman C, Ruedy KJ, Cheng P, Beck RW, for the Diabetes Research in Children Network (DirecNet). Evolution of abnormal plasma glucagon responses to mixed-meal feedings in youth with type 1 diabetes during the first 2 years after diagnosis. Diabetes Care. 2014;37(6):1741–4.
Michot J-M, Ragou P, Carbonnel F, Champiat S, Voisin A-L, Mateus C, Lambotte O, Annereau M. Significance of immune-related lipase increase induced by Antiprogrammed Death-1 or death Ligand-1 antibodies: a brief communication. J Immunother. 2018;41(2):84–5.
Yadav D, Nair S, Norkus EP, Pitchumoni CS. Nonspecific hyperamylasemia and hyperlipasemia in diabetic ketoacidosis: incidence and correlation with biochemical abnormalities. Am J Gastroenterol. 2000;95(11):3123–8.
Campbell-Thompson M, Rodriguez-Calvo T, Battaglia M. Abnormalities of the exocrine pancreas in type 1 diabetes. Curr Diab Rep. 2015;15(10):79.
Yoneda S, Imagawa A, Hosokawa Y, Baden MY, Kimura T, Uno S, Fukui K, Goto K, Uemura M, Eguchi H, Iwahashi H, Kozawa J, Shimomura I. T-lymphocyte infiltration to islets in the pancreas of a patient who developed type 1 diabetes after administration of immune checkpoint inhibitors. Diabetes Care. 2019;42(7):e116–8.
Taniguchi T, Okazaki K, Okamoto M, Seko S, Tanaka J, Uchida K, et al. Prevalence of autoantibodies against carbonic anhydrase II and lactoferrin in type 1 diabetes: concept of autoimmune exocrinopathy and endocrinopathy of the pancreas. Pancreas. 2003;27(1):26–30.
Hardt PD, Ewald N, Bröckling K, Tanaka S, Endo T, Kloer HU, Bretzel RG, Jaeger C, Shimura H, Kobayashi T. Distinct autoantibodies against exocrine pancreatic antigens in European patients with type 1 diabetes mellitus and non-alcoholic chronic pancreatitis. JOP. 2008;9(6):683–9.
Endo T, Takizawa S, Tanaka S, Takahashi M, Fujii H, Kamisawa T, et al. Amylase -2A autoantibodies: novel marker of autoimmune pancreatitis and fulminant type 1 diabetes. Diabetes. 2009;58(3):732–7.
Falcao CK, Cabral MCS, Mota JM, Arbache ST, Costa-Riquetto AD, Muniz DQB, Cury-Martins J, Almeida MQ, Kaczemorska PC, Nery M, Teles MG. Acquired Lipodystrophy associated with Nivolumab in a patient with advanced renal cell carcinoma. J Clin Endocrinol Metab. 2019;104(8):3245–8.
Jehl A, Cugnet-Anceau C, Vigouroux C, Legeay AL, Dalle S, Harou O, Marchand L, Lascols O, Caussy C, Thivolet C, Laville M, Disse E. Acquired generalized lipodystrophy: a new cause of anti-PD-1 immune-related diabetes. Diabetes Care. 2019;42(10):2008–10.
Judd J, Zibelman M, Handorf E, O’Neill J, Ramamurthy C, Bentota S, et al. Immune-related adverse events as a biomarker in non-melanoma patients treated with programmed cell death 1 inhibitors. Oncologist. 2017;22(10):1232–7.
Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22(4):886–94.
Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, et al. NCCN guidelines insights: management of immunotherapy-related toxicities, Version 1. J Natl Compr Cancer Netw. 2020;18(3):230–41.
Kong SH, Lee SY, Yang YS, Kim TM, Kwak SH. Anti-programmed cell death 1 therapy triggering diabetic ketoacidosis and fulminant type 1 diabetes. Acta Diabetol. 2016;53(5):853–6.
Sothornwit J, Phunmanee A, Pongchaiyakul C. Atezolizumab-induced autoimmune diabetes in a patient with metastatic lung cancer. Front Endocrinol. 2019;10:352.
Mellati M, Eaton KD, Brooks-Worrell BM, Hagopian WA, Martins R, Palmer JP, Hirsch IB. Anti–PD-1 and anti–PDL-1 monoclonal antibodies causing type 1 diabetes. Diabetes Care. 2015;38(9):e137–8.
Maekawa T, Okada K, Okada H, Kado S, Kamiya K, Komine M, Murata S, Oka K, Ishibashi S, Ohtsuki M. Case of acute-onset type 1 diabetes induced by Long-term immunotherapy with Nivolumab in a patient with mucosal melanoma. J Dermatol. 2019;46(12):e463–4.
Lee S, Morgan A, Shah S, Ebeling PR. Rapid-onset diabetic ketoacidosis secondary to Nivolumab therapy. Endocrinol Diabetes Metab Case Rep. 2018; pii: 18-0021
Acknowledgements
We would like to thank Anna Buganè for her contribution to this manuscript, and Adrian Wallwork for English revision (E4AC English for Academics).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have nothing to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lo Preiato, V., Salvagni, S., Ricci, C. et al. Diabetes mellitus induced by immune checkpoint inhibitors: type 1 diabetes variant or new clinical entity? Review of the literature. Rev Endocr Metab Disord 22, 337–349 (2021). https://doi.org/10.1007/s11154-020-09618-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-020-09618-w