Abstract
Carcinoid syndrome represents the most common functional syndrome that affects patients with neuroendocrine neoplasms. Its clinical presentation is really heterogeneous, ranging from mild and often misdiagnosed symptoms to severe manifestations, that significantly worsen the patient’s quality of life, such as difficult-to-control diarrhoea and fibrotic complications. Serotonin pathway alteration plays a central role in the pathophysiology of carcinoid syndrome, accounting for most clinical manifestations and providing diagnostic tools. Serotonin pathway is complex, resulting in production of biologically active molecules such as serotonin and melatonin, as well as of different intermediate molecules and final metabolites. These activities require site- and tissue-specific catalytic enzymes. Variable expression and activities of these enzymes result in different clinical pictures, according to primary site of origin of the tumour. At the same time, the biochemical diagnosis of carcinoid syndrome could be difficult even in case of typical symptoms. Therefore, the accuracy of the diagnostic methods of assessment should be improved, also attenuating the impact of confounding factors and maybe considering new serotonin precursors or metabolites as diagnostic markers. Finally, the prognostic role of serotonin markers has been only evaluated for its metabolite 5-hydroxyindole acetic acid but, due to heterogeneous and biased study designs, no definitive conclusions have been achieved. The most recent progress is represented by the new therapeutic agent telotristat, an inhibitor of the enzyme tryptophan hydroxylase, which blocks the conversion of tryptophan in 5-hydroxy-tryptophan. The present review investigates the clinical significance of serotonin pathway in carcinoid syndrome, considering its role in the pathogenesis, diagnosis, prognosis and therapy.

Similar content being viewed by others
Abbreviations
- 5-HIA:
-
5-Hydroxyindole Acetaldehyde
- 5-HIAA:
-
5-Hydroxyindole Acetic Acid
- 5-HTP:
-
5-Hydroxytryptophan
- GTOL:
-
5-Hydroxytryptophol Glucuronide
- 5-HTOL:
-
5-Hydroxytryptophol
- ADH:
-
Alcohol Dehydrogenase
- ALR:
-
Aldehyde Reductase
- AADC:
-
Aromatic Acid Decarboxylase
- CHD:
-
Carcinoid Heart Disease
- CaS:
-
Carcinoid Syndrome
- CNS:
-
Central Nervous System
- EC :
-
Enterochromaffin Cell
- HPLC :
-
High-Performance Liquid Chromatography
- HIOMT :
-
Hydroxyindole O-Methyltransferase
- TRP :
-
L-Tryptophan
- TPH :
-
L-Tryptophan-5-Hydroxilase
- LC :
-
Liquid Chromatography
- MS :
-
Mass Spectrometry
- MEL:
-
Melatonin
- MAO :
-
Monoamine Oxidase
- NAS :
-
N-Acetyl-Serotonin
- NEN:
-
Neuroendocrine Neoplasm
- SERT :
-
Serotonin Transporter
- 5-HT :
-
Serotonin, 5-Hydroxytryptamine
- SNAT :
-
Serotonin-N-Acetyltransferase
- TE:
-
Telotristat etiprate
- UGT:
-
UDP-Glucuronosyltransferase
- U-5-HIAA:
-
Urinary 5-Hydroxyindole Acetic Acid
- VMATs:
-
Vesicular Monoamine Transporters
References
Thorson A, Biorck G, Bjorkman G, Waldenstrom J. Malignant carcinoid of the small intestine with metastases to the liver, valvular disease of the right side of the heart (pulmonary stenosis and tricuspid regurgitation without septal defects), peripheral vasomotor symptoms, bronchoconstriction, and an unusual type of cyanosis; a clinical and pathologic syndrome. Am Heart J. 1954;47(5):795–817.
Kronenberg HWR. Williams textbook of endocrinology, vol. IXI. 11th ed; 2008.
Levine RJ, Elsas LJ, Duvall CP, Rice JS. Malignant carcinoid tumors with and without Flushing. JAMA. 1963;186:905–7.
Feldman JM. Carcinoid tumors and syndrome. Semin Oncol. 1987;14(3):237–46.
Beri N, Farid A, Galkin M, Lewis W, Amsterdam E. Diagnostic Dilemma: Carcinoid Syndrome. Am J Med. 2018;131(10):e405–e7. https://doi.org/10.1016/j.amjmed.2018.05.026.
Halperin DM, Shen C, Dasari A, Xu Y, Chu Y, Zhou S, et al. Frequency of carcinoid syndrome at neuroendocrine tumour diagnosis: a population-based study. Lancet Oncol. 2017;18(4):525–34. https://doi.org/10.1016/S1470-2045(17)30110-9.
Pandit S, Bhusal K. Carcinoid syndrome. Treasure Island: StatPearls; 2019.
Ito T, Igarashi H, Jensen RT. Pancreatic neuroendocrine tumors: clinical features, diagnosis and medical treatment: advances. Best Pract Res Clin Gastroenterol. 2012;26(6):737–53. https://doi.org/10.1016/j.bpg.2012.12.003.
Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD. Current status of gastrointestinal carcinoids. Gastroenterology. 2005;128(6):1717–51.
Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66(9):1673–80.
Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299(5603):76. https://doi.org/10.1126/science.1078197.
Lesurtel M, Soll C, Graf R, Clavien PA. Role of serotonin in the hepato-gastroIntestinal tract: an old molecule for new perspectives. Cell Mol Life Sci. 2008;65(6):940–52. https://doi.org/10.1007/s00018-007-7377-3.
Suominen T, Uutela P, Ketola RA, Bergquist J, Hillered L, Finel M, et al. Determination of serotonin and dopamine metabolites in human brain microdialysis and cerebrospinal fluid samples by UPLC-MS/MS: discovery of intact Glucuronide and sulfate conjugates. PLoS One. 2013;8(6):e68007. https://doi.org/10.1371/journal.pone.0068007.
Kvols LK. Metastatic carcinoid tumors and the malignant carcinoid syndrome. Ann N Y Acad Sci. 1994;733:464–70.
Bender DA. Biochemistry of tryptophan in health and disease. Mol Asp Med. 1983;6(2):101–97.
Feldman JM. Serotonin metabolism in patients with carcinoid tumors: incidence of 5-hydroxytryptophan-secreting tumors. Gastroenterology. 1978;75(6):1109–14.
Kang H, O'Connell JB, Leonardi MJ, Maggard MA, McGory ML, Ko CY. Rare tumors of the colon and rectum: a national review. Int J Color Dis. 2007;22(2):183–9. https://doi.org/10.1007/s00384-006-0145-2.
Cunningham JL, Janson ET, Agarwal S, Grimelius L, Stridsberg M. Tachykinins in endocrine tumors and the carcinoid syndrome. Eur J Endocrinol. 2008;159(3):275–82. https://doi.org/10.1530/EJE-08-0196.
Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–72. https://doi.org/10.1200/JCO.2007.15.4377.
Sherman SK, Maxwell JE, O'Dorisio MS, O'Dorisio TM, Howe JR. Pancreastatin predicts survival in neuroendocrine tumors. Ann Surg Oncol. 2014;21(9):2971–80. https://doi.org/10.1245/s10434-014-3728-0.
von der Ohe MR, Camilleri M, Kvols LK, Thomforde GM. Motor dysfunction of the small bowel and colon in patients with the carcinoid syndrome and diarrhea. N Engl J Med. 1993;329(15):1073–8. https://doi.org/10.1056/NEJM199310073291503.
Boutzios G, Kaltsas G. Clinical syndromes related to gastrointestinal neuroendocrine neoplasms. Front Horm Res. 2015;44:40–57. https://doi.org/10.1159/000382053.
Oberg K, Theodorsson-Norheim E, Norheim I. Motilin in plasma and tumor tissues from patients with the carcinoid syndrome. Possible involvement in the increased frequency of bowel movements. Scand J Gastroenterol. 1987;22(9):1041–8.
Hannah-Shmouni F, Stratakis CA, Koch CA. Flushing in (neuro)endocrinology. Rev Endocr Metab Disord. 2016;17(3):373–80. https://doi.org/10.1007/s11154-016-9394-8.
Connolly HM, Pellikka PA. Carcinoid heart disease. Curr Cardiol Rep. 2006;8(2):96–101.
Modlin IM, Shapiro MD, Kidd M. Carcinoid tumors and fibrosis: an association with no explanation. Am J Gastroenterol. 2004;99(12):2466–78. https://doi.org/10.1111/j.1572-0241.2004.40507.x.
Oberg K, Casanovas O, Castano JP, Chung D, Delle Fave G, Denefle P, et al. Molecular pathogenesis of neuroendocrine tumors: implications for current and future therapeutic approaches. Clin Cancer Res. 2013;19(11):2842–9. https://doi.org/10.1158/1078-0432.CCR-12-3458.
Ito T, Lee L, Jensen RT. Carcinoid-syndrome: recent advances, current status and controversies. Curr Opin Endocrinol Diabetes Obes. 2018;25(1):22–35. https://doi.org/10.1097/MED.0000000000000376.
Aluri V, Dillon JS. Biochemical testing in neuroendocrine tumors. Endocrinol Metab Clin N Am. 2017;46(3):669–77. https://doi.org/10.1016/j.ecl.2017.04.004.
Meijer WG, Kema IP, Volmer M, Willemse PH, de Vries EG. Discriminating capacity of indole markers in the diagnosis of carcinoid tumors. Clin Chem. 2000;46(10):1588–96.
Kema IP, Meijer WG, Meiborg G, Ooms B, Willemse PH, de Vries EG. Profiling of tryptophan-related plasma indoles in patients with carcinoid tumors by automated, on-line, solid-phase extraction and HPLC with fluorescence detection. Clin Chem. 2001;47(10):1811–20.
Oberg K, Modlin IM, De Herder W, Pavel M, Klimstra D, Frilling A, et al. Consensus on biomarkers for neuroendocrine tumour disease. Lancet Oncol. 2015;16(9):e435–e46. https://doi.org/10.1016/S1470-2045(15)00186-2.
Oberg K, Couvelard A, Delle Fave G, Gross D, Grossman A, Jensen RT, et al. ENETS consensus guidelines for standard of Care in Neuroendocrine Tumours: biochemical markers. Neuroendocrinology. 2017;105(3):201–11. https://doi.org/10.1159/000472254.
Kuo TR, Chen JS, Chiu YC, Tsai CY, Hu CC, Chen CC. Quantitative analysis of multiple urinary biomarkers of carcinoid tumors through gold-nanoparticle-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal Chim Acta. 2011;699(1):81–6. https://doi.org/10.1016/j.aca.2011.05.012.
Tellez MR, Mamikunian G, O'Dorisio TM, Vinik AI, Woltering EA. A single fasting plasma 5-HIAA value correlates with 24-hour urinary 5-HIAA values and other biomarkers in midgut neuroendocrine tumors (NETs). Pancreas. 2013;42(3):405–10. https://doi.org/10.1097/MPA.0b013e318271c0d5.
Adaway JE, Dobson R, Walsh J, Cuthbertson DJ, Monaghan PJ, Trainer PJ, et al. Serum and plasma 5-hydroxyindoleacetic acid as an alternative to 24-h urine 5-hydroxyindoleacetic acid measurement. Ann Clin Biochem. 2016;53(Pt 5):554–60. https://doi.org/10.1177/0004563215613109.
O'Toole D, Grossman A, Gross D, Delle Fave G, Barkmanova J, O'Connor J, et al. ENETS consensus guidelines for the standards of Care in Neuroendocrine Tumors: biochemical markers. Neuroendocrinology. 2009;90(2):194–202. https://doi.org/10.1159/000225948.
Rubin de Celis Ferrari AC, Glasberg J, Riechelmann RP. Carcinoid syndrome: update on the pathophysiology and treatment. Clinics (Sao Paulo). 2018;73(suppl 1):e490s. https://doi.org/10.6061/clinics/2018/e490s.
Corcuff JB, Chardon L, El Hajji RI, Brossaud J. Urinary sampling for 5HIAA and metanephrines determination: revisiting the recommendations. Endocr Connect. 2017;6(6):R87–98. https://doi.org/10.1530/EC-17-0071.
Gallo M, Muscogiuri G, Pizza G, Ruggeri RM, Barrea L, Faggiano A, et al. The management of neuroendocrine tumours: a nutritional viewpoint. Crit Rev Food Sci Nutr. 2019;59(7):1046–57. https://doi.org/10.1080/10408398.2017.1390729.
Swami T, Weber HC. Updates on the biology of serotonin and tryptophan hydroxylase. Curr Opin Endocrinol Diabetes Obes. 2018;25(1):12–21. https://doi.org/10.1097/MED.0000000000000383.
Kanakis G, Kaltsas G. Biochemical markers for gastroenteropancreatic neuroendocrine tumours (GEP-NETs). Best Pract Res Clin Gastroenterol. 2012;26(6):791–802. https://doi.org/10.1016/j.bpg.2012.12.006.
Kyriacou A, Stepien KM, Issa B. Urinary steroid metabolites in a case of florid Ectopic Cushing's syndrome and clinical correlations. Hormones (Athens). 2016;15(4):540–7. https://doi.org/10.14310/horm.2002.1695.
Gill GV, Yong A, Power E, Ramage J. Carcinoid-associated ectopic ACTH syndrome with variable response to octreotide. Postgrad Med J. 1999;75(880):98–100. https://doi.org/10.1136/pgmj.75.880.98.
Isidori AM, Venneri MA, Graziadio C, Simeoli C, Fiore D, Hasenmajer V, et al. Effect of once-daily, modified-release hydrocortisone versus standard glucocorticoid therapy on metabolism and innate immunity in patients with adrenal insufficiency (DREAM): a single-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2018;6(3):173–85. https://doi.org/10.1016/S2213-8587(17)30398-4.
Pozza C, Graziadio C, Giannetta E, Lenzi A, Isidori AM. Management strategies for aggressive Cushing's syndrome: from macroadenomas to Ectopics. J Oncol. 2012;2012:685213. https://doi.org/10.1155/2012/685213.
Kema IP, de Vries EG, Slooff MJ, Biesma B, Muskiet FA. Serotonin, catecholamines, histamine, and their metabolites in urine, platelets, and tumor tissue of patients with carcinoid tumors. Clin Chem. 1994;40(1):86–95.
Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, et al. The human serum metabolome. PLoS One. 2011;6(2):e16957. https://doi.org/10.1371/journal.pone.0016957.
Bouatra S, Aziat F, Mandal R, Guo AC, Wilson MR, Knox C, et al. The human urine metabolome. PLoS One. 2013;8(9):e73076. https://doi.org/10.1371/journal.pone.0073076.
Gijsman HJ, van Gerven JM, de Kam ML, Schoemaker RC, Pieters MS, Weemaes M, et al. Placebo-controlled comparison of three dose-regimens of 5-hydroxytryptophan challenge test in healthy volunteers. J Clin Psychopharmacol. 2002;22(2):183–9.
Guo K, Li L. Differential 12C-/13C-isotope dansylation labeling and fast liquid chromatography/mass spectrometry for absolute and relative quantification of the metabolome. Anal Chem. 2009;81(10):3919–32. https://doi.org/10.1021/ac900166a.
Trabado S, Al-Salameh A, Croixmarie V, Masson P, Corruble E, Feve B, et al. The human plasma-metabolome: reference values in 800 French healthy volunteers; impact of cholesterol, gender and age. PLoS One. 2017;12(3):e0173615. https://doi.org/10.1371/journal.pone.0173615.
Alfredsson G, Wiesel FA. Monoamine metabolites and amino acids in serum from schizophrenic patients before and during sulpiride treatment. Psychopharmacology. 1989;99(3):322–7.
Yao JK, Dougherty GG Jr, Reddy RD, Keshavan MS, Montrose DM, Matson WR, et al. Altered interactions of tryptophan metabolites in first-episode neuroleptic-naive patients with schizophrenia. Mol Psychiatry. 2010;15(9):938–53. https://doi.org/10.1038/mp.2009.33.
Lentner C. Geigy Scientific Tables. 8th Rev ed. Switzerland c; 1981–1992. p. 165–77.
Beck O, Borg S, Jonsson G, Lundman A, Valverius P. Measurement of 5-hydroxytryptophol and 5-hydroxyindoleacetic acid in human and rat brain and plasma. J Neural Transm. 1984;59(1):57–67.
Fuertig R, Ceci A, Camus SM, Bezard E, Luippold AH, Hengerer B. LC-MS/MS-based quantification of kynurenine metabolites, tryptophan, monoamines and neopterin in plasma, cerebrospinal fluid and brain. Bioanalysis. 2016;8(18):1903–17. https://doi.org/10.4155/bio-2016-0111.
Sakaguchi Y, Ikenaga J, Yoshida H, Hayama T, Itoyama M, Todoroki K, et al. Selective and sensitive liquid chromatographic determination method of 5-hydroxyindoles with fluorous and fluorogenic derivatization. J Pharm Biomed Anal. 2015;114:348–54. https://doi.org/10.1016/j.jpba.2015.06.003.
Boulet L, Faure P, Flore P, Monteremal J, Ducros V. Simultaneous determination of tryptophan and 8 metabolites in human plasma by liquid chromatography/tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2017;1054:36–43. https://doi.org/10.1016/j.jchromb.2017.04.010.
Tekes K. HPLC determination of serotonin and its metabolites from human platelet-rich plasma; shift to 5-hydroxytryptophol formation following alcohol consumption. J Chromatogr Sci. 2008;46(2):169–73. https://doi.org/10.1093/chromsci/46.2.169.
Miller AG, Brown H, Degg T, Allen K, Keevil BG. Measurement of plasma 5-hydroxyindole acetic acid by liquid chromatography tandem mass spectrometry--comparison with HPLC methodology. J Chromatogr B Anal Technol Biomed Life Sci. 2010;878(7–8):695–9. https://doi.org/10.1016/j.jchromb.2010.01.010.
Stephanson N, Helander A, Beck O. Alcohol biomarker analysis: simultaneous determination of 5-hydroxytryptophol glucuronide and 5-hydroxyindoleacetic acid by direct injection of urine using ultra-performance liquid chromatography-tandem mass spectrometry. J Mass Spectrom. 2007;42(7):940–9. https://doi.org/10.1002/jms.1231.
Carter MD, Calcutt MW, Malow BA, Rose KL, Hachey DL. Quantitation of melatonin and n-acetylserotonin in human plasma by nanoflow LC-MS/MS and electrospray LC-MS/MS. J Mass Spectrom. 2012;47(3):277–85. https://doi.org/10.1002/jms.2051.
Wolrab D, Fruhauf P, Gerner C. Quantification of the neurotransmitters melatonin and N-acetyl-serotonin in human serum by supercritical fluid chromatography coupled with tandem mass spectrometry. Anal Chim Acta. 2016;937:168–74. https://doi.org/10.1016/j.aca.2016.08.012.
Yang S, Zheng X, Xu Y, Zhou X. Rapid determination of serum melatonin by ESI-MS-MS with direct sample injection. J Pharm Biomed Anal. 2002;30(3):781–90.
Borucki K, Schreiner R, Dierkes J, Jachau K, Krause D, Westphal S, et al. Detection of recent ethanol intake with new markers: comparison of fatty acid ethyl esters in serum and of ethyl glucuronide and the ratio of 5-hydroxytryptophol to 5-hydroxyindole acetic acid in urine. Alcohol Clin Exp Res. 2005;29(5):781–7.
Stephanson N, Dahl H, Helander A, Beck O. Determination of urinary 5-hydroxytryptophol glucuronide by liquid chromatography-mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2005;816(1–2):107–12. https://doi.org/10.1016/j.jchromb.2004.11.026.
Wiseman JM, Puolitaival SM, Takats Z, Cooks RG, Caprioli RM. Mass spectrometric profiling of intact biological tissue by using desorption electrospray ionization. Angew Chem Int Ed Eng. 2005;44(43):7094–7. https://doi.org/10.1002/anie.200502362.
Gerbig S, Golf O, Balog J, Denes J, Baranyai Z, Zarand A, et al. Analysis of colorectal adenocarcinoma tissue by desorption electrospray ionization mass spectrometric imaging. Anal Bioanal Chem. 2012;403(8):2315–25. https://doi.org/10.1007/s00216-012-5841-x.
Phelps DL, Balog J, Gildea LF, Bodai Z, Savage A, El-Bahrawy MA, et al. The surgical intelligent knife distinguishes normal, borderline and malignant gynaecological tissues using rapid evaporative ionisation mass spectrometry (REIMS). Br J Cancer. 2018;118(10):1349–58. https://doi.org/10.1038/s41416-018-0048-3.
Davis Z, Moertel CG, McIlrath DC. The malignant carcinoid syndrome. Surg Gynecol Obstet. 1973;137(4):637–44.
Agranovich AL, Anderson GH, Manji M, Acker BD, Macdonald WC, Threlfall WJ. Carcinoid tumour of the gastrointestinal tract: prognostic factors and disease outcome. J Surg Oncol. 1991;47(1):45–52.
Wangberg B, Westberg G, Tylen U, Tisell L, Jansson S, Nilsson O, et al. Survival of patients with disseminated midgut carcinoid tumors after aggressive tumor reduction. World J Surg. 1996;20(7):892–9 discussion 9.
Onaitis MW, Kirshbom PM, Hayward TZ, Quayle FJ, Feldman JM, Seigler HF, et al. Gastrointestinal carcinoids: characterization by site of origin and hormone production. Ann Surg. 2000;232(4):549–56.
Hellman P, Lundstrom T, Ohrvall U, Eriksson B, Skogseid B, Oberg K, et al. Effect of surgery on the outcome of midgut carcinoid disease with lymph node and liver metastases. World J Surg. 2002;26(8):991–7. https://doi.org/10.1007/s00268-002-6630-z.
van der Horst-Schrivers AN, Post WJ, Kema IP, Links TP, Willemse PH, Wymenga AN, et al. Persistent low urinary excretion of 5-HIAA is a marker for favourable survival during follow-up in patients with disseminated midgut carcinoid tumours. Eur J Cancer. 2007;43(18):2651–7. https://doi.org/10.1016/j.ejca.2007.07.025.
Formica V, Wotherspoon A, Cunningham D, Norman AR, Sirohi B, Oates J, et al. The prognostic role of WHO classification, urinary 5-hydroxyindoleacetic acid and liver function tests in metastatic neuroendocrine carcinomas of the gastroenteropancreatic tract. Br J Cancer. 2007;96(8):1178–82. https://doi.org/10.1038/sj.bjc.6603699.
Janson ET, Holmberg L, Stridsberg M, Eriksson B, Theodorsson E, Wilander E, et al. Carcinoid tumors: analysis of prognostic factors and survival in 301 patients from a referral center. Ann Oncol. 1997;8(7):685–90.
Turner GB, Johnston BT, McCance DR, McGinty A, Watson RG, Patterson CC, et al. Circulating markers of prognosis and response to treatment in patients with midgut carcinoid tumours. Gut. 2006;55(11):1586–91. https://doi.org/10.1136/gut.2006.092320.
Bergestuen DS, Aabakken L, Holm K, Vatn M, Thiis-Evensen E. Small intestinal neuroendocrine tumors: prognostic factors and survival. Scand J Gastroenterol. 2009;44(9):1084–91. https://doi.org/10.1080/00365520903082432.
Zandee WT, Kamp K, van Adrichem RC, Feelders RA, de Herder WW. Limited value for urinary 5-HIAA excretion as prognostic marker in gastrointestinal neuroendocrine tumours. Eur J Endocrinol. 2016;175(5):361–6. https://doi.org/10.1530/EJE-16-0392.
Zandee WT, van Adrichem RC, Kamp K, Feelders RA, van Velthuysen MF, de Herder WW. Incidence and prognostic value of serotonin secretion in pancreatic neuroendocrine tumours. Clin Endocrinol. 2017;87(2):165–70. https://doi.org/10.1111/cen.13364.
Ghia JE, Li N, Wang H, Collins M, Deng Y, El-Sharkawy RT, et al. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology. 2009;137(5):1649–60. https://doi.org/10.1053/j.gastro.2009.08.041.
Manocha M, Khan WI. Serotonin and GI disorders: an update on clinical and experimental studies. Clin Transl Gastroenterol. 2012;3:e13. https://doi.org/10.1038/ctg.2012.8.
Margolis KG, Stevanovic K, Li Z, Yang QM, Oravecz T, Zambrowicz B, et al. Pharmacological reduction of mucosal but not neuronal serotonin opposes inflammation in mouse intestine. Gut. 2014;63(6):928–37. https://doi.org/10.1136/gutjnl-2013-304901.
Kim JJ, Wang H, Terc JD, Zambrowicz B, Yang QM, Khan WI. Blocking peripheral serotonin synthesis by telotristat etiprate (LX1032/LX1606) reduces severity of both chemical- and infection-induced intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2015;309(6):G455–65. https://doi.org/10.1152/ajpgi.00299.2014.
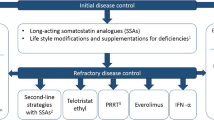
Kulke MH, O'Dorisio T, Phan A, Bergsland E, Law L, Banks P, et al. Telotristat etiprate, a novel serotonin synthesis inhibitor, in patients with carcinoid syndrome and diarrhea not adequately controlled by octreotide. Endocr Relat Cancer. 2014;21(5):705–14. https://doi.org/10.1530/ERC-14-0173.
Pavel M, Horsch D, Caplin M, Ramage J, Seufferlein T, Valle J, et al. Telotristat etiprate for carcinoid syndrome: a single-arm, multicenter trial. J Clin Endocrinol Metab. 2015;100(4):1511–9. https://doi.org/10.1210/jc.2014-2247.
Kulke MH, Horsch D, Caplin M, Anthony L, Bergsland E, Oberg K, et al. 37LBA Telotristat etiprate is effective in treating patients with carcinoid syndrome that is inadequately controlled by somatostatin analog therapy (the phase 3 TELESTAR clinical trial). 2015.
Pavel M, Gross DJ, Benavent M, Perros P, Srirajaskanthan R, Warner RRP, et al. Telotristat ethyl in carcinoid syndrome: safety and efficacy in the TELECAST phase 3 trial. Endocr Relat Cancer. 2018;25(3):309–22. https://doi.org/10.1530/ERC-17-0455.
Acknowledgements
This review is part of the ‘Neuroendocrine Tumours Innovation Knowledge and Education’ project led by Prof. Annamaria Colao and Prof. Antongiulio Faggiano, which aims at increasing the knowledge on neuroendocrine tumours. We would like to acknowledge all the Collaborators of this project: Manuela Albertelli, Barbara Altieri, Elena Ambrosetti, Antonio Bianchi, Lena Bottiglieri, Severo Campione, Silvia Carra, Federica De Cicco, Carla Di Dato, Sergio Di Molfetta, Antonella Di Sarno, Giuseppe Fanciulli, Diego Ferone, Francesco Ferraù, Marco Gallo, Elisa Giannetta, Federica Grillo, Elia Guadagno, Valentina Guarnotta, Andrea Isidori, Pasqualino Malandrino, Chiara Martini, Erika Messina, Roberta Modica, Giovanna Muscogiuri, Genoveffa Pizza, Paola Razzore, Laura Rizza, Manila Rubino, Concetta Sciammarella, Giovanni Vitale, Maria Chiara Zatelli.
Authorship
All authors made substantial contributions to the conception of the article, literature search and data analysis. The manuscript was drafted by Giuseppe Fanciulli and Rosaria M. Ruggeri and all authors commented on previous versions of the manuscript. Antongiulio Faggiano, Andrea Isidori and Annamaria Colao critically revised the work for important intellectual content. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fanciulli, G., Ruggeri, R.M., Grossrubatscher, E. et al. Serotonin pathway in carcinoid syndrome: Clinical, diagnostic, prognostic and therapeutic implications. Rev Endocr Metab Disord 21, 599–612 (2020). https://doi.org/10.1007/s11154-020-09547-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-020-09547-8