Abstract
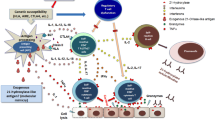
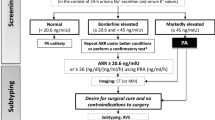
Less than 15% of hypertension cases in children are secondary to a primary hyperaldosteronism. This is idiopathic in 60% of the cases, secondary to a unilateral adenoma in 30% and 10% remaining by primary adrenal hyperplasia, familial hyperaldosteronism, ectopic aldosterone production or adrenocortical carcinoma.To date, four types of familial hyperaldosteronism (FH I to FH IV) have been reported. FH III is caused by germline mutations in KCNJ5, encoding the potassium channel Kir3.4. The mutations cause the channel to lose its selectivity for potassium, allowing large quantities of sodium to enter the cell. As a consequence, the membrane depolarizes, voltage-gated calcium channels open, calcium enters the cell, initiating the cascade that leads to aldosterone synthesis. Somatic mutations in KCNJ5 has also been described in aldosterone-producing adenomas. The most frequent presentation of FH III is with severe hyperaldosteronism symptoms and resistance to pharmacological therapy which leads to bilateral adrenalectomy. We will review current literature and describe a child with FH III due to a novel de novo deletion in KCNJ5 with wild phenotype as a sign of clinical variability of this disease.



Similar content being viewed by others
Abbreviations
- FH:
-
Familial hyperaldosteronism
- PA:
-
Primary aldosteronism
- APA:
-
Aldosterone producing adenoma
- BAH:
-
Bilateral adrenal hyperplasia
- ACTH:
-
Adrenocorticotropic hormone
- BP:
-
Blood pressure
- SDS:
-
Standard deviation
References
Thomas RM, Ruel E, Shantavasinkul PC, Corsino L. Endocrine hypertension: an overview on the current etiopathogenesis and management options. World J Hypertens. 2015;5:14–27.
Young WF, Sterns RH. Familial hyperaldosteronism. 2018 in https://www.uptodate.com/contents/familial-hyperaldosteronism. Accessed 21 Aug 2018
Lifton RP, Dluhy RG, Powers M, Rich GM, Cook S, Ulick S, et al. A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature. 1992;355:262–5.
Mulatero P, di Cella SM, Monticone S, Schiavone D, Manzo M, Mengozzi G, et al. 18-hydroxycorticosterone, 18-hydroxycortisol, and 18-oxocortisol in the diagnosis of primary aldosteronism and its subtypes. J Clin Endocrinol Metab. 2012;97:881–9.
Mulatero P, Tizzani D, Viola A, Bertello C, Monticone S, Mengozzi G, et al. Prevalence and characteristics of familial hyperaldosteronism: the PATOGEN study (primary Aldosteronism in TOrino-GENetic forms). Hypertension. 2011;58:797–803.
Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889–916. https://doi.org/10.1210/jc.2015-4061.
Gordon RD, Stowasser M, Tunny TJ, Klemm SA, Finn WL, Krek AL. Clinical and pathological diversity of primary aldosteronism, including a new familial variety. Clin Exp Pharmacol Physiol. 1991;18:283–6.
Sukor N, Mulatero P, Gordon RD, So A, Duffy D, Bertello C, et al. Further evidence for linkage of familial hyperaldosteronism type II at chromosome 7p22 in Italian as well as Australian and South American families. J Hypertens. 2008;26:1577–82.
Geller DS, Zhang J, Wisgerhof MV, Shackleton C, Kashgarian M, Lifton RP. A novel form of human mendelian hypertension featuring nonglucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab. 2008;93:3117–23.
Choi M, Scholl UI, Yue P, Bjorklund P, Zhao B, Nelson-Williams C, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331:768–72.
Monticone S, Hattangady NG, Penton D, Isales CM, Edwards MA, Williams TA, et al. A novel Y152C KCNJ5 mutation responsible for familial hyperaldosteronism type III. J Clin Endocrinol Metab. 2013;98:E1861–5.
Monticone S, Bandulik S, Stindl J, Zilbermint M, Dedov I, Mulatero P, et al. A case of severe hyperaldosteronism caused by a de novo mutation affecting a critical salt bridge Kir3.4 residue. J Clin Endocrinol Metab. 2015;100:E114–8.
Galati SJ, Hopkins SM, Cheesman KC, Zhuk RA, Levine AC. Primary aldosteronism: emerging trends. Trends Endocrinol Metab. 2013;24:421–30.
Monticone S, Tetti M, Burrello J, Buffolo F, de Giovanni R, Veglio F, et al. Familial hyperaldosteronism type III. J Hum Hypertens. 2017;31:776–81.
Scholl UI, Stolting G, Nelson-Williams C, et al. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. Elife. 2015;4:e06315.
Monticone S, Buffolo F, Tetti M, Veglio F, Pasini B, Mulatero P. Genetics in endocrinology: the expanding genetic horizon of primary aldosteronism. Eur J Endocrinol. 2018;178:R101–11.
Fernandes-Rosa FL, Williams TA, Riester A, Steichen O, Beuschlein F, Boulkroun S, et al. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension. 2014;64:354–61.
Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:3266–81.
Douillard C, Houillier P, Nussberger J, Girerd X. SFE/SFHTA/AFCE consensus on primary Aldosteronism, part 2: first diagnostic steps. Ann Endocrinol (Paris). 2016;77:192–201.
Martinez-Aguayo A, Aglony M, Campino C, Garcia H, Bancalari R, Bolte L, et al. Aldosterone, plasma renin activity, and aldosterone/renin ratio in a normotensive healthy pediatric population. Hypertension. 2010;56:391–6.
Amar L, Baguet JP, Bardet S, Chaffanjon P, Chamontin B, Douillard C, et al. SFE/SFHTA/AFCE primary aldosteronism consensus: introduction and handbook. Ann Endocrinol (Paris). 2016;77:179–86.
Reznik Y, Amar L, Tabarin A. SFE/SFHTA/AFCE consensus on primary aldosteronism, part 3: confirmatory testing. Ann Endocrinol (Paris). 2016;77:202–7.
Satoh F, Morimoto R, Ono Y, Iwakura Y, Omata K, Kudo M, et al. Measurement of peripheral plasma 18-oxocortisol can discriminate unilateral adenoma from bilateral diseases in patients with primary aldosteronism. Hypertension. 2015;65:1096–102.
Crudo V, Monticone S, Burrello J, Buffolo F, Tetti M, Veglio F, et al. Hyperaldosteronism: how to discriminate among different disease forms? High Blood Press Cardiovasc Prev. 2016;23:203–8.
Bardet S, Chamontin B, Douillard C, Pagny JY, Hernigou A, Joffre F, et al. SFE/SFHTA/AFCE consensus on primary aldosteronism, part 4: subtype diagnosis. Ann Endocrinol (Paris). 2016;77:208–13.
Rossi GP, Auchus RJ, Brown M, Lenders JWM, Naruse M, Plouin PF, et al. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension. 2014;63:151–60.
Pérez AJ, Casal M, Courel MA, Andrade MA. Hiperaldosteronismo primario: aspectos diagnósticos y terapéuticos. Hipertension y Riesgo Vascular. 2002;19:70–9.
Zennaro MC, Jeunemaitre X. SFE/SFHTA/AFCE consensus on primary aldosteronism, part 5: genetic diagnosis of primary aldosteronism. Ann Endocrinol (Paris). 2016;77:214–9.
Adachi M, Muroya K, Asakura Y, Sugiyama K, Homma K, Hasegawa T. Discordant genotype-phenotype correlation in familial hyperaldosteronism type III with KCNJ5 gene mutation: a patient report and review of the literature. Horm Res Paediatr. 2014;82:138–42.
Tong A, Liu G, Wang F, Jiang J, Yan Z, Zhang D, et al. A novel phenotype of familial hyperaldosteronism type III: concurrence of Aldosteronism and Cushing's syndrome. J Clin Endocrinol Metab. 2016;101:4290–7.
Gomez-Sanchez CE, Qi X, Gomez-Sanchez EP, Sasano H, Bohlen MO, Wisgerhof M. Disordered zonal and cellular CYP11B2 enzyme expression in familial hyperaldosteronism type 3. Mol Cell Endocrinol. 2017;439:74–80.
Nanba K, Omata K, Tomlins SA, Giordano TJ, Hammer GD, Rainey WE, et al. Double adrenocortical adenomas harboring independent KCNJ5 and PRKACA somatic mutations. Eur J Endocrinol. 2016;175:K1–6.
Kuppusamy M, Caroccia B, Stindl J, Bandulik S, Lenzini L, Gioco F, et al. A novel KCNJ5-insT149 somatic mutation close to, but outside, the selectivity filter causes resistant hypertension by loss of selectivity for potassium. J Clin Endocrinol Metab. 2014;99:E1765–73.
Zheng FF, Zhu LM, Nie AF, Li XY, Lin JR, Zhang K, et al. Clinical characteristics of somatic mutations in Chinese patients with aldosterone-producing adenoma. Hypertension. 2015;65:622–8.
Scholl UI, Healy JM, Thiel A, Fonseca AL, Brown TC, Kunstman JW, et al. Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype. Clin Endocrinol. 2015;83:779–89.
Nakamura Y, Yamazaki Y, Tezuka Y, Satoh F, Sasano H. Expression of CYP11B2 in aldosterone-producing adrenocortical adenoma: regulatory mechanisms and clinical significance. Tohoku J Exp Med. 2016;240:183–90.
Fernandes-Rosa FL, Boulkroun S, Zennaro MC. Somatic and inherited mutations in primary aldosteronism. J Mol Endocrinol. 2017;59:R47–63.
Pechere-Bertschi A, Herpin D, Lefebvre H. SFE/SFHTA/AFCE consensus on primary aldosteronism, part 7: medical treatment of primary aldosteronism. Ann Endocrinol (Paris). 2016;77:226–34.
Steichen O, Amar L, Chaffanjon P, Kraimps JL, Menegaux F, Zinzindohoue F. SFE/SFHTA/AFCE consensus on primary aldosteronism, part 6: adrenal surgery. Ann Endocrinol (Paris). 2016;77:220–5.
He BJ, Anderson ME. Aldosterone and cardiovascular disease: the heart of the matter. Trends Endocrinol Metab. 2013;24:21–30.
Baguet JP, Steichen O, Mounier-Vehier C, Gosse P. SFE/SFHTA/AFCE consensus on primary aldosteronism, part 1: epidemiology of PA, who should be screened for sporadic PA? Ann Endocrinol (Paris). 2016;77:187–91.
Tamargo J, Solini A, Ruilope LM. Comparison of agents that affect aldosterone action. Semin Nephrol. 2014;34:285–306.
Zennaro MC, Caprio M, Feve B. Mineralocorticoid receptors in the metabolic syndrome. Trends Endocrinol Metab. 2009;20:444–51.
Parviz Y, Iqbal J, Pitt B, Adlam D, Al-Mohammad A, Zannad F. Emerging cardiovascular indications of mineralocorticoid receptor antagonists. Trends Endocrinol Metab. 2015;26:201–11.
Author information
Authors and Affiliations
Contributions
Dr. Pons conceptualized the review, drafted the initial manuscript, and approved the final manuscript as submitted. Drs Moreno, Morata, Moriano, León, de Mingo and Calvo carried out the initial analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted. Dr. Zuñiga carried out the genetic test and approved the final manuscript.
Corresponding author
Ethics declarations
Financial disclosure
Dr. Pons and the remaining authors have no financial relationships relevant to this article to disclose.
Conflict of interest
Dr. Pons and the other authors have no conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Pons Fernández, N., Moreno, F., Morata, J. et al. Familial hyperaldosteronism type III a novel case and review of literature. Rev Endocr Metab Disord 20, 27–36 (2019). https://doi.org/10.1007/s11154-018-9481-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-018-9481-0