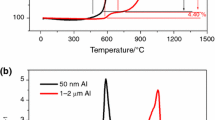
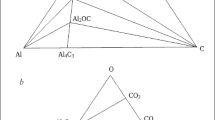
Ultradispersed high-purity aluminum nitride powder was obtained at the Physico-Technological Institute on a unique experimental-industrial unit by a gas-phase method. The powder was obtained through the formation of aluminum monofluoride. However, there has been very little thermodynamic analysis of the interactions of the materials in the reaction zone and in the charge. This study gives special attention to the selection of sintering additives and their effect on the removal of undesirable oxygen impurities during the synthesis of aluminum nitride. The mix that was obtained for sintering can be used for the energy-efficient single-stage production of aluminum-nitride finished products having excellent thermal conductivity.


Similar content being viewed by others
References
J. M. Lee, B. I. Kim, J. H. Lee, et al., “Low temperature synthesis of AlN nanopowders by microwave assisted combustion method,” J. Ceram. Processing Research, 14, No. 8, 707 – 711 (2013).
F. Liu, Z. J. Su, F. Y. Mo, et al., “Controlled synthesis of ultra-long AlN nanowires in different densities and in situ investigation of physical properties of an individual AlN nanowire,” Nanoscale, No. 3, 610 – 618 (2011).
Y. Tang, H. Cong, Z. Wang, et al., “Synthesis of rectangular cross-section AlN nanofibers by chemical vapor deposition,” Chemical Physics Letters, No. 416, 171 – 175 (2005).
A. A. Elagin, A. R. Beketov, M. V. Baranov, et al., “Aluminum nitride. Preparation methods (review),” Refractories and Industrial Ceramics, 53(6), 395 – 403 (2013).
A. A. Elagin, A. R. Beketov, M. V. Baranov, et al., “Aluminum nitride. Preparation methods,” Refractories and Industrial Ceramics, 54(1), 44 – 49 (2013).
A. A. Elagin, A. R. Beketov, M. V. Baranov, et al., “Mechanism of the process and technology for the gas-phase synthesis of aluminum nitride,” Sovr. Probl. Nauki i Obrazovaniya, No. 3 (2013).
U. S. Patent No. 4810679 A. Rare Earth Fluoride Additive for Sintering Aluminum Nitride (1989).
G. M. Gross, H. J. Seifert, and F. Aldinger, “Thermodynamic assessment and experimental check of fluoride sintering aids for AlN,” J. Europ. Ceram. Soc., No. 18, 871 – 877 (1998).
J. Jarridge, J. Mexmain, and J. P. Michelet, “Thermal diffusivity of AlN with fluoride additives,” 2nd Europ. Ceram. Soc. Conf. (ECSC). Augsburg (1991), pp. 1849 – 1854.
J. M. Dyke, C. Kirby, A. Morris, et al., “A study of aluminum monofluoride and aluminum trifluoride by high-temperature photoelectron-spectroscopy,” Chem. Phys., No. 88, 289 – 298 (1984).
Russian Federation Patent No. 2312060. Method of Obtaining Aluminum Nitride Powder. Yu. D. Afonin, A. R. Beketov, D. A. Beketov, and N. L. Chernyi. No. 2005102149. Sub. 28.01.05; Publ. 10.07.06.
A. A. Elagin, R. A. Shishkin, A. R. Beketov, and M. V. Baranov, “Thermodynamic analysis of the reactions in the production of aluminum nitride by a gas-phase method,” Sovr. Probl. Nauki i Obrazovaniya, No. 2 (2013) (htt: //www.science-education.ru/pdfi).
A. I. Belyaev and L. A. Firsanova, Monovalent Aluminum in Metallurgical Processes [in Russian], Metallurgizdat, Moscow (1952).
D. A. Beketov, A. R. Beketov, and L. B. Khoroshavin, “Composite coatings based on aluminum nitride and an organic binder,” Refractories and Industrial Ceramics, No. 44, 65 – 66 (2003).
V. P. Glushko, Thermodynamic Properties of Individual Substances [in Russian], Nauka, Moscow (1978).
L. V. Gurevich, Chemical Bond Energies. Ionization Potential and Electron Affinities of Elements: Handbook [in Russian], Nauka, Moscow (1974).
D. R. Stull and H. Prophet, Janaf Thermodinamical Tables, C-ov Print. off., Washington (1971), Vol. 24, No. 9, p. 1099.
I. S. Kulikov, Thermodynamics of Carbides and Nitrides, Metallurgiya, Chelyabinsk (1988).
A. L. Molisani, H. N. Yoshimura, and H. Goldenstein, “Effect of Y2O3 content on sintering of aluminum nitride,” Ceramica, 322(52), 151 – 160 (2006).
Y. Iwamoto, A. Kuibira, I. Sugiura, et al., “Effect of powder properties on thermal conductivity of aluminum nitride,” J. Ceram. Soc. Japan, 100(5), 652 – 656 (1992).
T. B. Troczynski, and P. S. Nicholson, “Effect of additives on the pressureless sintering of aluminum nitride between 1500-degrees and 1800-degrees-C,” J. Am. Ceram. Soc., 72(8), 1488 – 1491 (1989).
R. L. Diebner and J. G. Kay, “Absorption spectrum of vaporized titanium monofluoride,” J. Chem. Phys., 51(8), 3547 – 3554 (1969).
D. R. MacFarlane, P. J. Newman, and A. Voelkel, “Methods of purification of zirconium tetrafluoride for fluorozirconate glass,” J. Am. Ceram. Soc., 85(6), 1610 – 1612 (2002).
L. Rogstrom, N. Ghafoor, M. Ahlgren, et al., “Auto-organizing ZrAlN/ArAlTiN/TiN multilayers,” Thin Solid Films, No. 520, 6451 – 6454 (2012).
R. Franz, C. Lechthaler, C. Polzer, et al., “Oxidation behavior and tribological properties of arc-evaporated ZrAlN hard coatings,” Surface & Coating Technology, No. 206, 2337 – 2345 (2012).
M. Setoyama, A. Nakayama, M. Tanaka, et al., “Formation of cubic AlN in TiN/AlN superlattice,” Ibid., No. 86, 225 – 230 (1996).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Novye Ogneupory, No. 2, February, 2015.
Rights and permissions
About this article
Cite this article
Shishkin, R.A., Elagin, A.A., Beketov, A.R. et al. Thermodynamic Analysis of a New Gas-Phase Method of Obtaining High-Purity Aluminum Nitride. Refract Ind Ceram 56, 97–102 (2015). https://doi.org/10.1007/s11148-015-9790-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11148-015-9790-8