Abstract
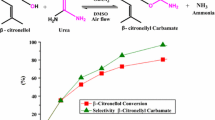
In this work, inexpensive and simple commercial transition metal salts were evaluated as catalysts in the acetalization of alkyl alcohols with β-citronellal, a renewable origin substrate. After an initial screening, FeCl3 was the most active and selective catalyst among the various transition metal salts evaluated toward the β-citronellal methyl acetal. The impacts of main reaction parameters such as time, temperature, catalyst load, and type of alcohol on conversion and selectivity of the reactions were investigated. Different iron salts were also investigated. It was demonstrated that both oxidation number and type of anion present in the salt play an essential role in this reaction. Notably, the dissolution of catalyst salts in solution triggered a decrease in the pH of the medium due to the hydrolysis (and or solvolysis) of the metal cation, impacting the conversion and reaction selectivity. The highest activity of FeCl3 was assigned to the greatest Lewis acidity strength, as demonstrated by the acidity measurements. This inexpensive, low-corrosive, and commercially affordable catalyst has advantages over traditional liquid mineral acid catalysts and provides an alternative route to synthesize alkyl terpene acetals.










Similar content being viewed by others
References
Lenardão EJ, Botteselle GV, De Azambuja F, Perin G, Jaco RG (2007) Citronellal as a key compound in organic synthesis. Tetrahedron 63:6671–6712. https://doi.org/10.1016/j.tet.2007.03.159
Tsolakis N, Bam W, Srai JS, Kumar M (2019) Renewable chemical feedstock supply network design: The case of terpenes. J Clean Prod 222:802–822. https://doi.org/10.1016/j.jclepro.2019.02.108
Wu L, Moteki T, Gokhale AA, Flaherty DW, Toste FD (2016) Production of fuels and chemicals from biomass: condensation reactions and beyond. Chem 1:32–58. https://doi.org/10.1016/j.chempr.2016.05.002
Sanchez LM, Thomas HJ, Climent MJ et al (2016) Heteropolycompounds as catalysts for biomass product transformations. Catal Rev 58:497–586. https://doi.org/10.1080/01614940.2016.1248721
Hamada N, Kazahaya K, Shimizu H, Sato T (2004) An efficient and versatile procedure for the synthesis of acetals from aldehydes and ketones catalyzed by lithium tetrafluoroborate. Synlett. https://doi.org/10.1055/s-2004-820038
Dong J-L, Yu L-S-H, Xie J-W (2018) A Simple and versatile method for the formation of Acetals/Ketals Using trace conventional acids. ACS Omega 3:4974–4985. https://doi.org/10.1021/acsomega.8b00159
Corma A, García H (2003) Lewis acids: from conventional homogeneous to green homogeneous and heterogeneous catalysis. Chem Rev 103:4307–4366. https://doi.org/10.1021/cr030680z
Wegenhart BL, Liu S, Thom M, Stanley D, Abu-Omar MM (2012) Solvent-free methods for making acetals derived from glycerol and furfural and their use as a biodiesel fuel component. ACS Catal 2:2524–2530. https://doi.org/10.1021/cs300562e
Umbarkar SB, Kotbagi TV, Biradar AV, Pasricha R, Chanale J, Dongare MK, Mamede A-S, Lancelot C, Payen E (2009) Acetalization of glycerol using mesoporous MoO3/SiO2 solid acid catalyst. J Mol Catal A 310:150–158. https://doi.org/10.1016/j.molcata.2009.06.010
Leonard NM, Oswald MC, Freiberg DA, Nattier BA, Smith RC, Mohan RS (2002) A simple and versatile method for the synthesis of Acetals from aldehydes and ketones using bismuth triflate. J Org Chem 67:5202–5207. https://doi.org/10.1021/jo0258249
Smith BM, Graham AE (2011) Indium triflate mediated tandem acetalisation-acetal exchange reactions under solvent-free conditions. Tetrahedron Lett 52:6281–6283. https://doi.org/10.1016/j.tetlet.2011.09.087
Smith BM, Kubczyk TM, Graham AE (2012) Indium(III) triflate catalysed transacetalisation reactions of diols and triols under solvent-free conditions. Tetrahedron 68:7775–7781. https://doi.org/10.1016/j.tet.2012.07.048
Cooks RG, Chen H, Eberlin MN, Zheng X, Tao WA (2006) Polar acetalization and transacetalization in the gas phase: the Eberlin reaction. Chem Rev 106:188–211. https://doi.org/10.1021/cr0400921
Zhu Z, Espenson JH (1997) Organometallic catalysis: formation of 1,3-dioxolanes and their analogs catalyzed by methylrhenium trioxide (MTO). Organometallics 16:3658–3663. https://doi.org/10.1021/om970225r
Clerici A, Pastori N, Porta O (2001) Mild acetalisation of mono and dicarbonyl compounds catalysed by titanium tetrachloride. Facile synthesis of β-keto enol ethers. Tetrahedron 57:217–225. https://doi.org/10.1016/S0040-4020(00)01001-2
da Silva MJ, de Oliveira CM (2021) Metal nitrate-catalyzed one-pot oxidative esterification of benzaldehyde with hydrogen peroxide in alcoholic solutions at room temperature. New J Chem 45:3683–3691. https://doi.org/10.1039/D0NJ05671E
da Silva MJ, Ayala DAM (2016) Unravelling transition metal-catalyzed terpenic alcohol esterification: a straightforward process for the synthesis of fragrances. Catal Sci Technol 6:3197–3207. https://doi.org/10.1039/C5CY01538C
Martins FP, Rodrigues FA, da Silva MJ (2018) Fe2(SO4)3-catalyzed Levulinic acid esterification: production of fuel bioadditives. Energies 11:1263. https://doi.org/10.3390/en11051263
da Silva MJ, Julio AA, Ayala DAM, de Miranda LMP (2018) Fe2(SO4)3-catalyzed synthesis of terpenic alcohols esters: a simple and bifunctional reusable solid catalyst. ChemSelect 3:5742–5748. https://doi.org/10.1002/slct.201800643
da Silva MJ, Carari DM, da Silva AM (2015) Fe(III)-catalyzed α-terpinyl derivatives synthesis from β-pinene via reactions with hydrogen peroxide in alcoholic solutions. RSC Adv 5:10529–10536. https://doi.org/10.1039/C4RA13112F
Carari DM, da Silva MJ (2014) Fe(NO3)3-catalyzed monoterpene oxidation by hydrogen peroxide: an inexpensive and environmentally benign oxidative process. Catal Lett 144:615–622. https://doi.org/10.1007/s10562-013-1189-x
da Silva MJ, Teixeira MG (2018) Assessment on the double role of the transition metal salts on the acetalization of furfural: Lewis and Brønsted acid catalysts. Mol Catal 461:40–47. https://doi.org/10.1016/j.mcat.2018.10.002
da Silva M, Silva G, Sampaio V et al (2020) One-pot synthesis of benzaldehyde derivatives in PdCl2-catalyzed reactions with H2O2 in alcoholic solutions. Chem Pap. https://doi.org/10.1007/s11696-020-01408-7
da Silva MJ, de Ávila RF, Júlio AA (2017) SnF2-catalyzed glycerol ketalization: a friendly environmentally process to synthesize solketal at room temperature over on solid and reusable Lewis acid. Chem Engin J 307:828–835. https://doi.org/10.1016/j.cej.2016.09.002
Cardoso AL, Neves SCG, da Silva MJ (2009) Kinetic study of alcoholysis of the fatty acids catalyzed by tin chloride(II): an alternative catalyst for biodiesel production. Energ Fuels 23:1718–1722. https://doi.org/10.1021/ef800639h
Karamé I, Alamé M, Kanj A, Baydoun GN, Hazimeh H, el Masri M, Christ L (2011) Mild and efficient protection of diol and carbonyls as cyclic acetals catalysed by iron (III) chloride. C R Chim 14:525–529. https://doi.org/10.1016/j.crci.2010.12.001
Zaher S, Christ L, Abd El Rahim M, Kanj A, Karamé I (2017) Green acetalization of glycerol and carbonyl catalyzed by FeCl3·6H2O. Mol Catal 438:204–213. https://doi.org/10.1016/j.mcat.2017.06.006
da Silva MJ, Ribeiro CJA, Vilanculo CB (2023) How the content of protons and vanadium affects the activity of H3+nPMo12-nVnO40 (n = 0, 1, 2, or 3) catalysts on the oxidative esterification of benzaldehyde with hydrogen peroxide. Catal Lett 153:2045–2056. https://doi.org/10.1007/s10562-022-04132-x
da Silva MJ, Teixeira MG, Natalino R (2019) Highly selective synthesis under benign reaction conditions of furfural dialkyl acetal using SnCl2 as a recyclable catalyst. New J Chem 43:8606–8612. https://doi.org/10.1039/C9NJ01284B
Krompiec S, Penkala M, Szczubiałka K, Kowalska E (2012) Transition metal compounds and complexes as catalysts in the synthesis of acetals and orthoesters: theoretical, mechanistic and practical aspects. Coordin Chem Rev 256:2057–2095. https://doi.org/10.1016/j.ccr.2012.05.006
Teixeira MG, Natalino R, da Silva MJ (2020) A kinetic study of heteropolyacid-catalyzed furfural acetalization with methanol at room temperature via ultraviolet spectroscopy. Catal Today 344:143–149. https://doi.org/10.1016/j.cattod.2018.11.071
Acknowledgements
The authors are grateful for the financial support from CNPq and FAPEMIG (Brazil). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Funding
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior,001,Marcio Silva
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Venâncio, A.N., Ribeiro, C.J.A., Júlio, A.A. et al. Assessments on the transition metal salt-catalyzed β-citronellal condensation reactions with alkyl alcohols. Reac Kinet Mech Cat 137, 149–161 (2024). https://doi.org/10.1007/s11144-023-02528-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02528-3