Abstract
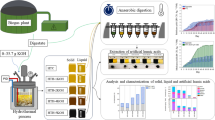
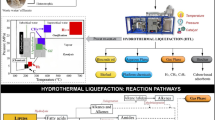
This study investigates the production of furfural from flax straw biomass through hydrothermal hydrolysis using solid acid catalysts. Specifically, the potential of Glu–TsOH–Zr, Glu–TsOH–Ti, and Glu–TsOH catalysts in furfural production from flax straw biomass and pure xylose was examined. Various characterization techniques such as NH3-TPD, N2-Physisorption, XRD, SEM–EDS, and FTIR were employed to analyze the catalysts’ characteristics. The effect of reaction residence time (0–120 min) and reaction temperature (170–210 °C) on furfural yield was studied. Additionally, the optimal solvent ratio in a two-phase liquid system and the kinetics of the furfural production process were determined. Results showed that the Glu–TsOH–Zr catalyst exhibited increased furfural yield from flax straw without promoting furfural degradation. The catalyst capacity order in the hydrothermal hydrolysis process was found to be Glu–TsOH–Ti > Glu–TsOH–Zr > Glu–TsOH, and these catalysts yielded higher furfural from flax straw compared to previous studies. A first-order irreversible series reaction was identified for furfural production, with temperature having a greater impact on the reaction than the degradation process. The proposed model demonstrated a good fit, with an average absolute deviation of 5.9%. This study presents the kinetics and a comprehensive parametric analysis of a stable and selective sulfonated carbon-based zirconia catalyst for furfural production from flax straw, excluding xylose degradation products. The findings contribute to understanding the potential and limitations of flax straw liquefaction and provide insights for further advancements in catalyzed reactions for renewable fuel production.







Similar content being viewed by others
References
Machado G, Leon S, Santos F, Lourega R, Dullius J, Mollmann ME, Eichler P (2016) Literature review on furfural production from lignocellulosic biomass. Nat Resour 07:115–129
Jönsson LJ, Martín C (2016) Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour Technol 199:103–112
Harry I, Ibrahim H, Thring R, Idem R (2014) Catalytic subcritical water liquefaction of flax straw for high yield of furfural. Biomass Bioenergy 71:381–393
Li X, Mupondwa E, Panigrahi S, Tabil L, Sokhansanj S, Stumborg M (2012) A review of agricultural crop residue supply in Canada for cellulosic ethanol production. Renew Sustain Energy Rev 16:2954–2965
Rosales-Calderon O, Arantes V (2019) A review on commercial-scale high-value products that can be produced alongside cellulosic ethanol. Biotechnol Biofuels 12:240
Dulie NW, Woldeyes B, Demsash HD, Jabasingh AS (2021) An insight into the valorization of hemicellulose fraction of biomass into furfural: catalytic conversion and product separation. Waste Biomass Valorization 12:531–552
Harmsen P, Huijgen W, Bermudez L, Bakker R (2010) Literature review of physical and chemical pretreatment processes for lignocellulosic biomass. 54 Wageningen UR Food & Biobased Research. https://edepot.wur.nl/150289
Galbe M, Wallberg O (2019) Pretreatment for biorefineries: a review of common methods for efficient utilisation of lignocellulosic materials. Biotechnol Biofuels 12:294
Werpy T, Petersen G (2004) Top value added chemicals from biomass: volume I—results of screening for potential candidates from sugars and synthesis gas. DOE/GO-102004–1992, 15008859. https://doi.org/10.2172/15008859
Nhien LC, Long NVD, Kim S, Lee M (2017) Techno-economic assessment of hybrid extraction and distillation processes for furfural production from lignocellulosic biomass. Biotechnol Biofuels 10:81
Montané D, Salvadó J, Torras C, Farriol X (2002) High-temperature dilute-acid hydrolysis of olive stones for furfural production. Biomass Bioenergy 22:295–304
Barrett CJ, Chheda JN, Huber GW, Dumesic JA (2006) Single-reactor process for sequential aldol-condensation and hydrogenation of biomass-derived compounds in water. Appl Catal B 66:111–118
Jaafari L (2013) Catalytic production of furfural by the subcritical hydrothermal gasification of flax straw. MASc Thesis, FGSR, University of Regina. https://ourspace.uregina.ca/items/46ef06c3-d0b8-4d81-b045-fd7489b944d2
Zeitsch KJ (2000) The chemistry and technology of furfural and its many by-products. Elsevier, pp 36–74
Eken-Saraçoğlu N, Mutlu SF, Dilmaç G, Çavuşoğlu H (1998) A comparative kinetic study of acidic hemicellulose hydrolysis in corn cob and sunflower seed hull. Bioresour Technol 65:29–33
Mansilla HD, Baeza J, Urzúa S, Maturana G, Villaseñor J, Durán N (1998) Acid-catalysed hydrolysis of rice hull: evaluation of furfural production. Bioresour Technol 66:189–193
Lenihan P, Orozco A, O’Neill E, Ahmad MNM, Rooney DW, Walker GM (2010) Dilute acid hydrolysis of lignocellulosic biomass. Chem Eng J 156:395–403
Garrote G, Domı́nguez H, Parajó JC, (2001) Kinetic modelling of corncob autohydrolysis. Process Biochem 36:571–578
Karimi K, Kheradmandinia S, Taherzadeh MJ (2006) Conversion of rice straw to sugars by dilute-acid hydrolysis. Biomass Bioenergy 30:247–253
Mamman AS, Lee J-M, Kim Y-C, Hwang IT, Park N-J, Hwang YK, Chang J-S, Hwang J-S (2008) Furfural: hemicellulose/xylosederived biochemical. Biofuels Bioprod Biorefining 2:438–454
Möller M, Schröder U (2013) Hydrothermal production of furfural from xylose and xylan as model compounds for hemicelluloses. RSC Adv 3:22253–22260
Suxia R, Haiyan X, Jinling Z, Shunqing L, Xiaofeng H, Tingzhou L (2012) Furfural production from rice husk using sulfuric acid and a solid acid catalyst through a two-stage process. Carbohydr Res 359:1–6
Weidener D, Leitner W, Domínguez de María P, Klose H, Grande PM (2021) Lignocellulose fractionation using recyclable phosphoric acid: Lignin, cellulose, and furfural production. ChemSusChem 14:909–916
Rusanen A, Kupila R, Lappalainen K, Kärkkäinen J, Hu T, Lassi U (2020) Conversion of xylose to furfural over lignin-based activated carbon-supported iron catalysts. Catalysts 10:821
Dashtban M, Gilbert A, Fatehi P (2012) Production of furfural: overview and challenges. J Sci Technol For Prod Process 2:44–53
Chareonlimkun A, Champreda V, Shotipruk A, Laosiripojana N (2010) Catalytic conversion of sugarcane bagasse, rice husk and corncob in the presence of TiO2, ZrO2 and mixed-oxide TiO2–ZrO2 under hot compressed water (HCW) condition. Bioresour Technol 101:4179–4186
Chen X, Yang H, Chen Y, Chen W, Lei T, Zhang W, Chen H (2017) Catalytic fast pyrolysis of biomass to produce furfural using heterogeneous catalysts. J Anal Appl Pyrolysis 127:292–298
Wang W, Ren J, Li H, Deng A, Sun R (2015) Direct transformation of xylan-type hemicelluloses to furfural via SnCl4 catalysts in aqueous and biphasic systems. Bioresour Technol 183:188–194
Lin Q, Li H, Wang X, Jian L, Ren J, Liu C, Sun R (2017) SO42−/Sn-MMT solid acid catalyst for xylose and xylan conversion into furfural in the biphasic system. Catalysts 7:118
Zhang W, Wang Z, Huang J, Jiang Y (2021) Zirconia-based solid acid catalysts for biomass conversion. Energy Fuels 35:9209–9227
Gómez Millán G, Phiri J, Mäkelä M, Maloney T, Balu AM, Pineda A, Llorca J, Sixta H (2019) Furfural production in a biphasic system using a carbonaceous solid acid catalyst. Appl Catal Gen 585:117180
Verma S, Baig RBN, Nadagouda MN, Len C, Varma RS (2017) Sustainable pathway to furanics from biomass via heterogeneous organo-catalysis. Green Chem 19:164–168
Sairanen E, Vilonen K, Karinen R, Lehtonen J (2013) Functionalized activated carbon catalysts in xylose dehydration. Top Catal 56:512–521
Mazzotta MG, Gupta D, Saha B, Patra AK, Bhaumik A, Abu-Omar MM (2014) Efficient solid acid catalyst containing lewis and brønsted acid sites for the production of furfurals. ChemSusChem 7:2342–2350
Li W, Zhu Y, Lu Y, Liu Q, Guan S, Chang H, Jameel H, Ma L (2017) Enhanced furfural production from raw corn stover employing a novel heterogeneous acid catalyst. Bioresour Technol 245:258–265
Gómez Millán G, El Assal Z, Nieminen K, Hellsten S, Llorca J, Sixta H (2018) Fast furfural formation from xylose using solid acid catalysts assisted by a microwave reactor. Fuel Process Technol 182:56–67
Daengprasert W, Boonnoun P, Laosiripojana N, Goto M, Shotipruk A (2011) Application of sulfonated carbon-based catalyst for solvothermal conversion of cassava waste to hydroxymethylfurfural and furfural. Ind Eng Chem Res 50:7903–7910
Liang J, Zha J, Zhao N, Tang Z, He Y, Ma C (2021) Valorization of waste lignocellulose to furfural by sulfonated biobased heterogeneous catalyst using ultrasonic-treated chestnut shell waste as carrier. Processes 9:2269
Hu S, Huang J, Huang D, Li P, Tang J, Meng F (2021) Increased flexibility to improve the catalytic performance of carbon-based solid acid catalysts. Green Process Synth 10:687–699
Zhang B, Ren J, Liu X, Guo Y, Guo Y, Lu G, Wang Y (2010) Novel sulfonated carbonaceous materials from p-toluenesulfonic acid/glucose as a high-performance solid-acid catalyst. Catal Commun 11:629–632
Ogundowo F, Ibrahim H (2022) Promising sulfonated carbon-based zirconia catalyst for renewable furfural production. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-03071-2
Beyene D, Chae M, Vasanthan T, Bressler DC (2020) A biorefinery strategy that introduces hydrothermal treatment prior to acid hydrolysis for co-generation of furfural and cellulose nanocrystals. Front Chem 8:323
Wataniyakul P, Boonnoun P, Quitain AT, Sasaki M, Kida T, Laosiripojana N, Shotipruk A (2018) Preparation of hydrothermal carbon as catalyst support for conversion of biomass to 5-hydroxymethylfurfural. Catal Commun 104:41–47
Cai CM, Zhang T, Kumar R, Wyman CE (2014) Integrated furfural production as a renewable fuel and chemical platform from lignocellulosic biomass. J Chem Technol Biotechnol 89:2–10
Jing Q, Lü X (2007) Kinetics of non-catalyzed decomposition of D-xylose in high temperature liquid water* *supported by the national natural science foundation of China (No.20476089) and the project of the ministry of science and technology of China (No.2004CCA05500). Chin J Chem Eng 15:666–669
Sahu R, Dhepe PL (2012) A one-pot method for the selective conversion of hemicellulose from crop waste into C5 sugars and furfural by using solid acid catalysts. Chemsuschem 5:751–761
Vedernikovs N, Kampars V, Puķe M, Krūma I (2010) Changes in the birch wood lignocellulose composition in the pretreatment process. Mater Sci Appl Chem 22:68–73
Sun S, Cao X, Li H, Chen X, Tang J, Sun S (2018) Preparation of furfural from eucalyptus by the MIBK/H2O pretreatment with biphasic system and enzymatic hydrolysis of the resulting solid fraction. Energy Convers Manag 173:539–544
Ji H, Chen L, Zhu JY, Gleisner R, Zhang X (2016) Reaction kinetics based optimization of furfural production from corncob using a fully recyclable solid acid. Ind Eng Chem Res 55:11253–11259
Weingarten R, Cho J, Wm. Curtis Conner J, W. Huber G, (2010) Kinetics of furfural production by dehydration of xylose in a biphasic reactor with microwave heating. Green Chem 12:1423–1429
Li X, Liu Q, Luo C, Gu X, Lu L, Lu X (2017) Kinetics of furfural production from corn cob in γ-valerolactone using dilute sulfuric acid as catalyst. ACS Sustain Chem Eng 5:8587–8593
Funding
The financial supports of the Natural Sciences and Engineering Research Council of Canada (NSERC DG: RGPIN-2018-03955), Canada Foundation for Innovation (CFI JELF: 37758) the Vice President Research Office, and the Faculty of Engineering & Applied Science at the University of Regina provided as gratefully acknowledged. The authors are also grateful to the Clean Energy Technologies Research Institute (CETRI) for granting them access to their research facilities. The authors’ opinions are their own, not necessarily those of our research and funding partners.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ogundowo, O., Sadanandam, G. & Ibrahim, H. Furfural from flax straw using sulfonated carbonaceous acid catalyst: parametric and kinetic studies. Reac Kinet Mech Cat 136, 2535–2554 (2023). https://doi.org/10.1007/s11144-023-02466-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02466-0