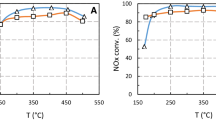
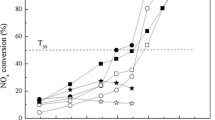
Abstract
Ba0.95La0.05FeO3 (BLF) was synthesized using a sol–gel method. The catalytic activity of the BLF catalyst towards the co-splitting of H2O/CO2 was investigated in a packed bed reactor and micro-channel reactor for comparison purposes. At the maximum reduction temperature of this study (700 °C), the oxygen vacancies of 3508 µmol/g were achieved. The H2O-TPSR and CO2-TPSR results revealed that H2O splitting took place at temperatures ranging from 400 to 700 °C while the CO2 splitting occurred at temperatures higher than 600 °C. The activation energy of H2O splitting and CO2 splitting was calculated at 23 and 124 kJ/mol, respectively. FTIR results suggested that the active hydroxyl group was responsible for H2O splitting, whereas the oxygen vacancy played a role in CO2 splitting. The micro-channel reactor was found to be advantageous for only the CO2 splitting. The hydroxyl groups were believed to be located on the catalyst’s surface, while the oxygen vacancies were more likely present within the catalyst’s bulk.










Similar content being viewed by others
Data availability
None.
References
Kunda D, Phiri H (2017) An approach for predicting CO2 emissions using data mining techniques. Int J Comput Appl 172:7–10
Wang F, Harindintwali JD, Yuan Z, Wang M, Wang F, Li S, Yin Z, Huang L, Fu Y, Li L, Chang SX, Zhang L, Rinklebe J, Yuan Z, Zhu Q, Xiang L, Tsang DCW, Xu L, Jiang X, Liu J, Wei N, Kastner M, Zou Y, Ok YS, Shen J, Peng D, Zhang W, Barceló D, Zhou Y, Bai Z, Li B, Zhang B, Wei K, Cao H, Tan Z, Zhao L, He X, Zheng J, Bolan N, Liu X, Huang C, Dietmann S, Luo M, Sun N, Gong J, Gong Y, Brahushi F, Zhang T, Xiao C, Li X, Chen W, Jiao N, Lehmann J, Zhu YG, Jin H, Schaffer A, Tiedje JM, Chen JM (2021) Technologies and perspectives for achieving carbon neutrality. Innovation 2(4):100180
Chen Y, Zhu X, Li K, Wei Y, Zheng Y, Wang H (2019) Chemical looping co-splitting of H2O-CO2 for efficient generation of syngas. ACS Sustain Chem Eng 7:15452–15462
Zhu X, Imtiaz Q, Donat F, Müller CR, Li F (2020) Chemical looping beyond combustion—a perspective. Energy Environ Sci 13:772–804
El-Nagar RA, Ghanem AA (2019) Syngas production, properties, and its importance, sustainable alternative syngas fuel. IntechOpen, pp 1–8
Lewis MA, Masin JG, O’Hare PA (2009) Evaluation of alternative thermochemical cycles, Part I: the methodology. Int J Hydrogen Energy 34:4115–4124
Luciani G, Landi G, Di Benedetto A (2020) Syngas production through H2O/CO2 thermochemical splitting over doped ceria-zirconia materials. Front Energy Res 8:204
Skinner SJ (2001) Recent advances in perovskite-type materials for solid oxide fuel cell cathodes. Int J Inorg Mater 3:113–121
Babu R, Vardhaman AK, Dhavale VM, Giribabu L, Singh SP (2019) MA2CoBr4: lead-free cobalt-based perovskite for electrochemical conversion of water to oxygen. Chem Commun 55:6779–6782
Demont A, Abanades S (2015) Solar thermochemical conversion of CO2 into fuel via two-step redox cycling of non-stoichiometric Mn-containing perovskite oxides. J Mater Chem A 3:3536–3546
Scheffe JR, Weibel D, Steinfeld A (2013) Lanthanum-strontium-manganese perovskites as redox materials for solar thermochemical splitting of H2O and CO2. Energy Fuels 27:4250–4257
McDaniel AH, Miller EC, Arifin D, Ambrosini A, Coker EN, O’Hayre R, Chueh WC, Tong J (2013) Sr- and Mn-doped LaAlO3 for solar thermochemical H2 and CO production. Energy Environ Sci 6:2424–2428
Takacs M, Hoes M, Caduff M, Cooper T, Scheffe JR, Steinfeld A (2015) Oxygen nonstoichiometry, defect equilibria, and thermodynamic characterization of LaMnO3 perovskites with Ca/Sr A-site and Al B-site doping. Acta Mater 103:700–710
Le Gal A, Abanades S, Flamant G (2011) CO2 and H2O splitting for thermochemical production of solar fuels using nonstoichiometric ceria and ceria/zirconia solid solutions. Energy Fuels 25:4836–4845
Demont A, Abanades S, Beche E (2014) Investigation of perovskite structures as oxygen-exchange redox materials for hydrogen production from thermochemical two-step water-splitting cycles. J Phys Chem C 118:12682–12692
Zou Y, Zhou W, Liu S, Shao Z (2011) Sintering and oxygen permeation studies of La0.6Sr0.4Co0.2Fe0.8O3-δ ceramic membranes with improved purity. J Eur Ceram Soc 31:2931–2938
Kida T, Takauchi D, Watanabe K, Yuasa M, Shimanoe K, Teraoka Y, Yamazoea N (2009) Oxygen permeation properties of partially A-site substituted BaFeO3-δ perovskites. J Electrochem Soc 156:E187–E191
Bedel L, Roger AC, Estournes C, Kiennemann A (2003) Co0 from partial reduction of La(Co, Fe)O3 perovskites for Fischer-Tropsch synthesis. Catal Today 85:207–218
Zhang C, Chang X, Fan Y, Jin W, Xu N (2007) Improving performance of a dense membrane reactor for thermal decomposition of CO2 via surface modification. Ind Eng Chem Res 46:2000–2005
Wang S, Xu J, Wu M, Song Z, Wang L, Zhang L, Yang J, Long W, Zhang L (2021) Cobalt–free perovskite cathode BaFe0.9Nb0.1O3-δ for intermediate-temperature solid oxide fuel cell. J Alloys Compd 872:159701
Lu Y, Zhao H, Chang X, Du X, Li K, Ma Y, Yi S, Du Z, Zheng K, Świerczek K (2016) Novel cobalt-free BaFe1-xGdxO3-δ perovskite membranes for oxygen separation. J Mater Chem A 4:10454–10466
Ngoensawat A, Tongnan V, Laosiripojana N, Kim-Lohsoontorn P, Hartley UW (2020) Effect of La and Gd substitution in BaFeO3-δ perovskite structure on its catalytic performance for thermochemical water splitting. Catal Commun 135:105901
Hayer F, Bakhtiary-davijany H, Myrstad R, Holmen A, Pfeifer P, Venvik HJ (2011) Synthesis of dimethyl ether from syngas in a microchannel reactor-Simulation and experimental study. Chem Eng J 167:610–615
Men Y, Kolb G, Zapf R, Hessel V, Löwe H (2007) Ethanol steam reforming in a microchannel reactor. Process Saf Environ Prot 85:413–418
Chen D, Chen C, Dong F, Shao Z, Ciucci F (2014) Cobalt-free polycrystalline Ba0.95La0.05FeO3−δ thin films as cathodes for intermediate-temperature solid oxide fuel cells. J Power Sources 250:188–195
Yoshimura M, Sardar K (2021) Revisiting the valence stability and preparation of perovskite structure type oxides ABO3 with the use of Madelung electrostatic potential energy and lattice site potential. RSC Adv 11:20737–20745
Polfus JM, Yildiz B, Tuller HL, Bredesen R (2018) Adsorption of CO2 and facile carbonate formation on BaZrO3 surfaces. J Phys Chem C 122:307–314
Petkovich ND, Rudisill SG, Venstrom LJ, Boman DB, Davidson JH, Stein A (2011) Control of heterogeneity in nanostructured Ce1−xZrxO2 binary oxides for enhanced thermal stability and water splitting activity. J Phys Chem C 115:21022–21033
Zhao Z, Uddi M, Tsvetkov N, Yildiz B, Ghoniem AF (2017) Enhanced intermediate-temperature CO2 splitting using nonstoichiometric ceria and ceria-zirconia. Phys Chem Chem Phys 19:25774–25785
Ngoenthong N, Hartley M, Sornchamni T, Siri-nguan N, Laosiripojana N, Hartley UW (2019) Comparison of packed-bed and micro-channel reactors for hydrogen production via thermochemical cycles of water splitting in the presence of ceria-based catalysts. Processes 7:767
Zhao Z, Uddi M, Tsvetkov N, Yildiz B, Ghoniem AF (2016) Redox kinetics study of fuel reduced ceria for chemical-looping water splitting. J Phys Chem C 120:16271–16289
Zhu X, Wang H, Wei Y, Li K, Cheng X (2011) Reaction characteristics of chemical-looping steam methane reforming over a Ce-ZrO2 solid solution oxygen carrier. Mendeleev Commun 21:221–223
Kang KS, Kim CH, Bae KK, Cho WC, Kim WJ, Kim YH, Kim SH, Park CS (2010) Redox cycling of CuFe2O4 supported on ZrO2 and CeO2 for two-step methane reforming/water splitting. Int J Hydrogen Energy 35:568–576
Ngoenthong N, Tongnan V, Sornchamni T, Siri-nguan N, Laosiripojana N, Hartley UW (2021) Application of a micro-channel reactor for process intensification in high purity syngas production via H2O/CO2 co-splitting. Int J Hydrogen Energy 46:24581–24590
Acknowledgements
This research was funded by KMUTNB (KMUTNB-FF-65-25 and KMUTNB-FF-66-52), Newton Fund through IAPP Thailand (TSP2021\100117), and NRCT (Contract Number N41A640149).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sereewatthanawut, I., Sornchamni, T., Siri-nguan, N. et al. Two-steps thermochemical cycles of H2O/CO2 co-splitting over Ba0.95La0.05FeO3 (BLF) in a packed bed reactor and micro-channel reactor. Reac Kinet Mech Cat 136, 1965–1981 (2023). https://doi.org/10.1007/s11144-023-02454-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02454-4