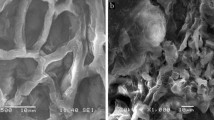
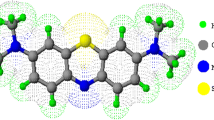
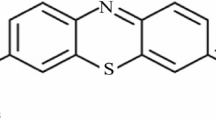
Abstract
This research investigates the potential of both Moringa Oleifera Leaves Green Powder (MOLGP) and its Base Activation Moringa Oleifera Leaves Green Powder (BAMOLGP) as a low-cost and efficient biosorbent for removing dye, metals, and bacteria from water. Specifically, safranin dye's adsorption capacity on MOLGP and BAMOLGP was examined, and MOLGP underwent base activation with sonication to enhance its adsorption capacity as a nanoparticle. The biosorbent surface characteristics were analyzed using FTIR, SEM, BET, and EDX techniques. XRD analysis confirmed the formation of a semi-crystalline form, and changes in surface morphology and elemental composition were observed after NaOH treatment. The maximum removal efficiency of safranin was 56.17% under the given conditions, but it significantly improved to 98.96% after undergoing treatments. The adsorption process was exothermic, and there was a decrease in system entropy during treatment. The results showed that safranin adsorption onto MOLGP was favorable enthalpy change and the entropy change was unfavorable at all temperatures, but adsorption onto BAMOLGP was favorable at all temperatures. Eleven statistical functions were employed to estimate the error deviations between experimental and theoretically predicted kinetic adsorption values and isothermals. The data indicated that the first order and second order equations best matched MOLOPG and BAMOLOPG, while Freundlich is the best match for isothermal BAMOLOPG.
Graphical Abstract









Similar content being viewed by others
References
Ugwu SN, Umuokoro AF, Echiegu EA, Ugwuishiwu BO, Enweremadu CC (2017) Comparative study of the use of natural and artificial coagulants for the treatment of sullage (domestic wastewater). Cogent Eng 4(1):1–13. https://doi.org/10.1080/23311916.2017.1365676
Nisar N, Koul B, Koul B (2020) Application of Moringa Oleifera Lam. seeds in wastewater treatment. Plant Arch 21:2408–2417. https://doi.org/10.51470/plantarchives.2021.v21.s1.393
Fayazi M, Afzali D, Taher MA, Mostafavi A, Gupta VK (2015) Removal of Safranin dye from aqueous solution using magnetic mesoporous clay: optimization study. J Mol Liq 212:675–685. https://doi.org/10.1016/j.molliq.2015.09.045
Ahmed H et al. (2023) Efficient removal of basic Fuchsin from synthetic medical wastewater and competitive adsorption in the mixture
Aldawsari AM, Alsohaimi I, Hassan HMA, Abdalla ZEA, Hassan I, Berber MR (2021) Tailoring an efficient nanocomposite of activated carbon-layered double hydroxide for elimination of water-soluble dyes. J Alloys Compd 857:157551. https://doi.org/10.1016/j.jallcom.2020.157551
Adesina OA, Taiwo AE, Akindele O, Igbafe A (2021) Process parametric studies for decolouration of dye from local ‘tie and dye’ industrial effluent using Moringa oleifera seed. South African J Chem Eng 37:23–30. https://doi.org/10.1016/j.sajce.2021.03.005
Saraei N, Ghazani MT (2021) Removal of acidic yellow dye of wastewater by Moringa Peregrina. pp. 1–26, 2021. Available: https://scholar.archive.org/work/p6huphhdkvhzvoonfrha5ajtdu/access/wayback/https://assets.researchsquare.com/files/rs-131703/v1_stamped.pdf
Gupta VK, Mittal A, Jain R, Mathur M, Sikarwar S (2006) Adsorption of Safranin-T from wastewater using waste materials- activated carbon and activated rice husks. J Colloid Interface Sci 303(1):80–86. https://doi.org/10.1016/j.jcis.2006.07.036
Gupta VK, Jain R, Mittal A, Mathur M, Sikarwar S (2007) Photochemical degradation of the hazardous dye Safranin-T using TiO2 catalyst. J Colloid Interface Sci 309(2):464–469. https://doi.org/10.1016/j.jcis.2006.12.010
Chowdhury S, Saha P (2011) Adsorption kinetic modeling of safranin onto rice husk biomatrix using pseudo-first- and pseudo-second-order kinetic models: comparison of linear and non-linear methods. Clean: Soil, Air, Water 39(3):274–282. https://doi.org/10.1002/clen.201000170
Preethi S, Sivasamy A, Sivanesan S, Ramamurthi V, Swaminathan G (2006) Removal of safranin basic dye from aqueous solutions by adsorption onto corncob activated carbon. Ind Eng Chem Res 45(22):7627–7632. https://doi.org/10.1021/ie0604122
Golder AK, Hridaya N, Samanta AN, Ray S (2005) Electrocoagulation of methylene blue and eosin yellowish using mild steel electrodes. J Hazard Mater 127(1–3):134–140. https://doi.org/10.1016/j.jhazmat.2005.06.032
Da̧browski A (2001) Adsorption–from theory to practice. Adv Colloid Interface Sci 93(1–3):135–224. https://doi.org/10.1016/S0001-8686(00)00082-8
Ahmed HR, Raheem SJ, Aziz BK (2017) Removal of Leishman stain from aqueous solutions using natural clay of Qulapalk area of Kurdistan region of Iraq. Karbala Int J Mod Sci 3(3):165–175. https://doi.org/10.1016/j.kijoms.2017.05.002
Aldawsari AM, Alsohaimi IH, Al-Kahtani AA, Alqadami AA, Ali Abdalla ZE, Saleh EAM (2021) Adsorptive performance of aminoterephthalic acid modified oxidized activated carbon for malachite green dye: mechanism, kinetic and thermodynamic studies. Sep Sci Technol 56(5):835–846. https://doi.org/10.1080/01496395.2020.1737121
Aldawsari AM et al (2020) Activated carbon/MOFs composite: AC/NH2-MIL-101(Cr), synthesis and application in high performance adsorption of p-nitrophenol. J Saudi Chem Soc 24(9):693–703. https://doi.org/10.1016/j.jscs.2020.07.009
Beltrán-Heredia J, Sánchez Martín J (2008) Azo dye removal by Moringa oleifera seed extract coagulation. Color Technol 124(5):310–317. https://doi.org/10.1111/j.1478-4408.2008.00158.x
Alosaimi EH, Hotan Alsohaimi I, Dahan TE, Chen Q, Melhi S (2021) Adsorptive performance of tetracarboxylic acid-modified magnetic silica nanocomposite for recoverable efficient removal of toxic Cd(II) from aqueous environment: equilibrium, isotherm, and reusability studies. J Mol Liq 334:116069. https://doi.org/10.1016/j.molliq.2021.116069
Gelebo GG, Ahmed FE (2019) Removal of direct and reactive dyes from textile wastewater using Moringa stenopetala seed extract. J Textile Eng Fashion Technol 5(4):184–191. https://doi.org/10.15406/jteft.2019.05.00200
El-Gaayda J et al (2022) “Optimization of turbidity and dye removal from synthetic wastewater using response surface methodology: Effectiveness of Moringa oleifera seed powder as a green coagulant. J Environ Chem Eng 10(1):106988. https://doi.org/10.1016/j.jece.2021.106988
Ncibi MC (2008) Applicability of some statistical tools to predict optimum adsorption isotherm after linear and non-linear regression analysis. J Hazard Mater 153(1–2):207–212. https://doi.org/10.1016/j.jhazmat.2007.08.038
Jasper EE, Ajibola VO, Onwuka JC (2020) Nonlinear regression analysis of the sorption of crystal violet and methylene blue from aqueous solutions onto an agro-waste derived activated carbon. Appl Water Sci 10(6):132. https://doi.org/10.1007/s13201-020-01218-y
Araújo CST, Melo EI, Alves VN, Coelho NMM (2010) Moringa oleifera Lam. seeds as a natural solid adsorbent for removal of AgI in aqueous solutions. J Braz Chem Soc 21(9):1727–1732. https://doi.org/10.1590/S0103-50532010000900019
Fatiqin A et al (2021) A comparative study on phytochemical screening and antioxidant activity of aqueous extract from various parts of Moringa oleifera. Indones J Nat Pigment 3(2):43. https://doi.org/10.33479/ijnp.2021.03.2.43
Simon S, Joseph SKJ, George D (2022) Optimization of extraction parameters of bioactive components from Moringa oleifera leaves using Taguchi method. Biomass Convers Biorefinery 1:4–8. https://doi.org/10.1007/s13399-021-02276-1
Yamaura M, Camilo RL, Sampaio LC, Macêdo MA, Nakamura M, Toma HE (2004) Preparation and characterization of (3-aminopropyl)triethoxysilane-coated magnetite nanoparticles. J Magn Magn Mater 279(2–3):210–217. https://doi.org/10.1016/j.jmmm.2004.01.094
Mnisi RL, Ndibewu PP (2017) Surface and adsorptive properties of Moringa oleifera bark for removal of V(V) from aqueous solutions. Environ Monit Assess 189(12):5. https://doi.org/10.1007/s10661-017-6329-0
Rezende C et al (2010) Characterization and use of Moringa oleifera seeds as biosorbent for removing metal ions from aqueous effluents. Water Sci Technol 15:2198–2203. https://doi.org/10.2166/wst.2010.419
Powder L, Techniques UT (2021) Characterization of novel solid dispersions of Moringa oleifera. Processes 9(12):2230
Jayan N, Bhatlu MLD, Akbar ST (2021) Central composite design for adsorption of Pb(II) and Zn(II) Metals on PKM-2 Moringa oleifera leaves. ACS Omega 6(39):25277–25298. https://doi.org/10.1021/acsomega.1c03069
Subramanyam B, Das A (2014) Linearised and non-linearised isotherm models optimization analysis by error functions and statistical means. J Environ Heal Sci Eng 12(1):92. https://doi.org/10.1186/2052-336X-12-92
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40(9):1361–1403. https://doi.org/10.1021/ja02242a004
Alqadami AA, Naushad M, Alothman ZA, Ahamad T (2018) Adsorptive performance of MOF nanocomposite for methylene blue and malachite green dyes: Kinetics, isotherm and mechanism. J Environ Manage 223(May):29–36. https://doi.org/10.1016/j.jenvman.2018.05.090
Hashem A, Al-Anwar A, Nagy NM, Hussein DM, Eisa S (2016) Isotherms and kinetic studies on adsorption of Hg(II) ions onto Ziziphus spina-christi L from aqueous solutions. Processes 5(2):213–224. https://doi.org/10.1515/gps-2015-0103
Funding
The author received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has stated that there are no potential conflicts of interest regarding the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmed, H.R., Radha, F.H.S., Agha, N.N.M. et al. Characterization and evaluation of Moringa Oleifera Leaves Green Powder and its alkali-activated form as eco-friendly biosorbent for the effective removal of safranin dye from synthetic wastewater. Reac Kinet Mech Cat 136, 2181–2201 (2023). https://doi.org/10.1007/s11144-023-02438-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02438-4