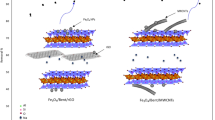
Abstract
Adsorption desulfurization of dibenzothiophene (DBT) in model oil was conducted in a batch-process and fixed-bed system by using four adsorbers: activated charcoal (AC), activated charcoal loaded with monometallic manganese (AC/Mn), activated charcoal loaded with monometallic copper (AC/Cu), and activated charcoal loaded with bimetallic manganese and copper (AC/Mn/Cu). The adsorbers are characterized by field emission scanning electron microscope (FESEM-EDS), X-ray diffraction analysis (XRD), Fourier transform infrared spectroscopy (FT-IR), Thermogravimetric analysis (TGA), and N2 adsorption–desorption analysis. The sequence AC/Mn/Cu > AC/Mn > AC/Cu > AC has been obtained from the results of removing dibenzothiophene (DBT) in the model oil in batch-process. The adsorption capacities (qe) and sulfur removal percentages were equal to (136.78 mg g−1, 91.18%), (95.85 mg g−1, 63.9%), (83.25 mg g−1, 55.5%), (70.8 mg g−1, 47.2%). Additionally, in the fixed-bed system, AC/Mn/Cu adsorber has shown the best performance compared to the three other adsorbers and it only lost 34% of its efficiency after 300 min. The adsorption process of DBT on AC/Mn/Cu adsorber followed Pseudo-Second-Order kinetics and the Langmuir adsorption isotherm model. In the adsorption desulfurization of high-sulfur commercial diesel fuel in a fixed-bed system by AC/Mn/Cu adsorber, DBT has been completely removed and 99% of total sulfur decreased to 16 ppm.
Graphical abstract






Similar content being viewed by others
References
Sadare OO, Obazu F, Daramola MO (2017) Biodesulfurization of petroleum distillates—current status, opportunities and future challenges. Environments 4(4):85
Wei Y et al (2018) Uncovering the culprits of air pollution: evidence from China’s economic sectors and regional heterogeneities. J Clean Prod 171:1481–1493
Rajendran A et al (2020) A comprehensive review on oxidative desulfurization catalysts targeting clean energy and environment. J MaterChem A 8(5):2246–2285
Ganiyu SA, Lateef SA (2021) Review of adsorptive desulfurization process: overview of the non-carbonaceous materials, mechanism and synthesis strategies. Fuel 294:120273
Shafiq I et al (2020) Recent developments in alumina supported hydrodesulfurization catalysts for the production of sulfur-free refinery products: a technical review. Catal Rev 64:1–86
Ahmadian M, Anbia M (2021) Oxidative desulfurization of liquid fuels using polyoxometalate-based catalysts: a review. Energy Fuels 35(13):10347–10373
Nassar HN, Abu Amr SS, El-Gendy NS (2021) Biodesulfurization of refractory sulfur compounds in petro-diesel by a novel hydrocarbon tolerable strain Paenibacillus glucanolyticus HN4. Environ Sci Pollut Res 28(7):8102–8116
Makoś P, Boczkaj G (2019) Deep eutectic solvents based highly efficient extractive desulfurization of fuels–Eco-friendly approach. J Mol Liq 296:111916
Zhang X-F et al (2018) Adsorptive desulfurization from the model fuels by functionalized UiO-66 (Zr). Fuel 234:256–262
Saha B, Vedachalam S, Dalai AK (2021) Review on recent advances in adsorptive desulfurization. Fuel Process Technol 214:106685
Ullah S et al (2020) Desulfurization of model oil through adsorption over activated charcoal and bentonite clay composites. Chem Eng Technol 43(3):564–573
Zhu L et al (2019) Modification of zeolite by metal and adsorption desulfurization of organic sulfide in natural gas. J Nat Gas Sci Eng 69:102941
Jiang B-L, Jiang N, Chang Y-X (2021) Synthesis of highly active Cu (I)-Y (III)-Y zeolite and its selective adsorption desulfurization performance in presence of xylene isomers. Pet Sci 18(1):295–306
Abedi MA, Abbasizadeh S, Karimzadeh R (2021) Adsorptive desulfurization of model diesel fuel over mono-functionalized nickel/γ-alumina and bi-functionalized nickel/cerium/γ-alumina adsorbents. Res Chem Intermed 47(2):497–520
Câmara AB et al (2020) Novel application for palygorskite clay mineral: a kinetic and thermodynamic assessment of diesel fuel desulfurization. Adsorption 26(2):267–282
Yosefi L, Khoshbin R, Karimzadeh R (2022) Beneficial incorporation of metal-sulfur interaction in adsorption capacity of boron nitride based adsorbents used in highly selective sulfur removal. Fuel 310:122277
Kampouraki Z-C et al (2019) Metal organic frameworks as desulfurization adsorbents of DBT and 4, 6-DMDBT from fuels. Molecules 24(24):4525
Jha D et al (2019) Adsorptive removal of dibenzothiophene from diesel fuel using microwave synthesized carbon nanomaterials. Fuel 244:132–139
Shi Y, Zhang X, Liu G (2015) Adsorptive desulfurization performances of ordered mesoporous carbons with tailored textural and surface properties. Fuel 158:565–571
Saleh TA, Danmaliki GI (2016) Influence of acidic and basic treatments of activated carbon derived from waste rubber tires on adsorptive desulfurization of thiophenes. J Taiwan Inst Chem Eng 60:460–468
Mguni LL et al (2019) Desulphurization of diesel fuels using intermediate Lewis acids loaded on activated charcoal and alumina. Chem Eng Commun 206(5):572–580
Saleh TA et al (2017) Adsorptive desulfurization of thiophene, benzothiophene and dibenzothiophene over activated carbon manganese oxide nanocomposite: with column system evaluation. J Clean Prod 154:401–412
Huo Q et al (2019) Cu, Zn-embedded MOF-derived bimetallic porous carbon for adsorption desulfurization. Chem Eng J 378:122106
Shah SS, Ahmad I, Ahmad W (2016) Adsorptive desulphurization study of liquid fuels using Tin (Sn) impregnated activated charcoal. J Hazard Mater 304:205–213
Ganiyu SA et al (2016) Influence of aluminium impregnation on activated carbon for enhanced desulfurization of DBT at ambient temperature: role of surface acidity and textural properties. Chem Eng J 303:489–500
Azeez MO et al (2022) Synergistic effect of nitrogen and molybdenum on activated carbon matrix for selective adsorptive desulfurization: Insights into surface chemistry modification. Arab J Chem 15(1):103454
Yaseen M et al (2021) Fabrication of Zn and Mn loaded activated carbon derived from corn cobs for the adsorptive desulfurization of model and real fuel oils. Fuel 284:119102
Danmaliki GI, Saleh TA (2017) Effects of bimetallic Ce/Fe nanoparticles on the desulfurization of thiophenes using activated carbon. Chem Eng J 307:914–927
Saleh TA (2018) Simultaneous adsorptive desulfurization of diesel fuel over bimetallic nanoparticles loaded on activated carbon. J Clean Prod 172:2123–2132
Saleh TA et al (2018) Ultra-deep adsorptive desulfurization of fuels on cobalt and molybdenum nanoparticles loaded on activated carbon derived from waste rubber. J Colloid Interface Sci 513:779–787
Shu J et al (2019) Fractional removal of manganese and ammonia nitrogen from electrolytic metal manganese residue leachate using carbonate and struvite precipitation. Water Res 153:229–238
Chen W-S, Liao C-T, Lin K-Y (2017) Recovery zinc and manganese from spent battery powder by hydrometallurgical route. Energy Procedia 107:167–174
Taylor A, Frazier A, Gurney E (1963) Solubility products of magnesium ammonium and magnesium potassium phosphates. Trans Faraday Soc 59:1580–1584
Mirbagheri SA, Hosseini SN (2005) Pilot plant investigation on petrochemical wastewater treatmentfor the removal of copper and chromium with the objective of reuse. Desalination 171(1):85–93
Qin X et al. (2018) Investigation of plating wastewater treatment technology for chromium, nickel and copper. In IOP Conference Series: Earth and Environmental Science. IOP Publishing
Cairns MJ et al (2006) A study of the uptake of copper ions by nanostructured calcium silicate. Microporous Mesoporous Mater 95(1–3):126–134
Zhang Q et al (2021) An experimental study of Ni-Mo adsorbent for reactive adsorption desulfurization of spent tire pyrolysis oil modelled using n-hexane and thiophene. Fuel 303:121272
Mirhoseini H, Taghdiri M (2016) Extractive oxidation desulfurization of sulfur-containing model fuel using hexamine–phosphotungstate hybrid as effective heterogeneous catalyst. Fuel 167:60–67
Seredych M, Bandosz TJ (2010) Adsorption of dibenzothiophenes on activated carbons with copper and iron deposited on their surfaces. Fuel Process Technol 91(6):693–701
Chen Q, Zhang L, Chen G (2012) Facile preparation of graphene-copper nanoparticle composite by in situ chemical reduction for electrochemical sensing of carbohydrates. Anal Chem 84(1):171–178
Mironova-Ulmane N et al (2018) Synthesis and vibration spectroscopy of nano-sized manganese oxides. Acta Physica Polonica Series A. https://doi.org/10.12693/APhysPolA.133.1013
Swetha B, Geetha A (2014) Synthesis and characterization of nickel oxide, manganese oxide nanoparticles and NiO/MnO Nano composite: hydrothermal approach. ChemTech Res 7:2138–2143
Bazrafshan A, Hajati S, Ghaedi M (2015) Synthesis of regenerable Zn (OH) 2 nanoparticle-loaded activated carbon for the ultrasound-assisted removal of malachite green: optimization, isotherm and kinetics. RSC Adv 5(96):79119–79128
Obeidat S, Hammoudeh A, Alomary A (2018) Application of FTIR spectroscopy for assessment of green coffee beans according to their origin. J Appl Spectrosc 84(6):1051–1055
Zhu G-Z et al (2016) Comparative study on characterization and adsorption properties of activated carbons by phosphoric acid activation from corncob and its acid and alkaline hydrolysis residues. Fuel Process Technol 144:255–261
Hudgins DM, Allamandola L (1997) Infrared spectroscopy of matrix-isolated polycyclic aromatic hydrocarbon cations. 4 The tetracyclic PAH isomers chrysene and 1, 2-benzanthracene. J Phys Chem A 101(19):3472–3477
Dashamiri S et al (2016) Ultrasonic enhancement of the simultaneous removal of quaternary toxic organic dyes by CuO nanoparticles loaded on activated carbon: central composite design, kinetic and isotherm study. Ultrason Sonochem 31:546–557
Elango M et al (2018) Synthesis, characterization, and antibacterial activity of polyindole/Ag–Cuo nanocomposites by reflux condensation method. Polym-Plast Technol Eng 57(14):1440–1451
Nieto-Delgado C, Terrones M, Rangel-Mendez J (2011) Development of highly microporous activated carbon from the alcoholic beverage industry organic by-products. Biomass Bioenerg 35(1):103–112
Bardestani R, Patience GS, Kaliaguine S (2019) Experimental methods in chemical engineering: specific surface area and pore size distribution measurements—BET, BJH, and DFT. Can J Chem Eng 97(11):2781–2791
Sing K, Rouqerol J, Siemieniewska T (1985) Pure Appl Chem 57:603
Kaneko K (1994) Determination of pore size and pore size distribution: 1. Adsorbents and catalysts. J Membr Sci 96(1–2):59–89
Bae Y-S, Yazaydın AO, Snurr RQ (2010) Evaluation of the BET method for determining surface areas of MOFs and zeolites that contain ultra-micropores. Langmuir 26(8):5475–5483
Kim KC, Yoon T-U, Bae Y-S (2016) Applicability of using CO2 adsorption isotherms to determine BET surface areas of microporous materials. Microporous Mesoporous Mater 224:294–301
Wan Mokhtar WNA et al (2015) Catalytic oxidative desulfurization of diesel oil by Co/Mn/Al2O3 catalysts—tert-butyl hydroperoxide (TBHP) system: preparation, characterization, reaction, and mechanism. Clean Technol Environ Policy 17(6):1487–1497
Ayers PW (2007) The physical basis of the hard/soft acid/base principle. Faraday Discuss 135:161–190
Ayers PW (2005) An elementary derivation of the hard/soft-acid/base principle. J Chem Phys 122(14):141102
Menzel R et al (2016) Graphene oxide/mixed metal oxide hybrid materials for enhanced adsorption desulfurization of liquid hydrocarbon fuels. Fuel 181:531–536
Samarghandi MR, Hadi M, McKay G (2014) Breakthrough curve analysis for fixed-bed adsorption of Azo dyes using novel pine cone—derived active carbon. Adsorpt Sci Technol 32(10):791–806
Zou W, Bai H, Gao S (2012) Competitive adsorption of neutral Red and Cu2+ onto pyrolytic char: isotherm and kinetic study. J Chem Eng Data 57(10):2792–2801
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I. Solids. J Am Chem Soc 38(11):2221–2295
Weber TW, Chakravorti RK (1974) Pore and solid diffusion models for fixed-bed adsorbers. AIChE J 20(2):228–238
Freundlich H (1906) Over the adsorption in solution. J Phys chem 57(385471):1100–1107
Tran HN et al (2017) Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: a critical review. Water Res 120:88–116
Jafarinasab M et al (2020) An efficient Co-based metal–organic framework nanocrystal (Co-ZIF-67) for adsorptive desulfurization of dibenzothiophene: Impact of the preparation approach on structure tuning. Energy Fuels 34(10):12779–12791
Lagergren S (1898) About the theory of so-called adsorption of solution substances. pp. 147–156
Blanchard G, Maunaye M, Martin G (1984) Removal of heavy metals from waters by means of natural zeolites. Water Res 18(12):1501–1507
Acknowledgements
The authors would like to acknowledge the Department of Chemistry, at the University of Sulaimani for the opportunity to conduct this research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there are no conflicts with this manuscript. They have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Othman, C.S., Salih, Y.M., Hamasalih, L.O. et al. Influence of different nanocomposite carbon-based adsorbers on the adsorption desulfurization of dibenzothiophene in model oil and diesel fuel: a comparative study. Reac Kinet Mech Cat 136, 919–936 (2023). https://doi.org/10.1007/s11144-023-02378-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02378-z