Abstract
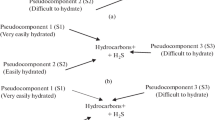
Using NiMo/γ-Al2O3 catalyst and a pilot-scale unit, a kinetic model was developed for the second reactor of the two-stage hydrogenation process to describe the HDS behavior of diesel in sulfur content lower than 10 μg g−1. The HDS reaction happened in the second reactor was simulated by combining the mass transfer, kinetics model and physical property data of diesel, the results show that the simulated values of sulfur content were within an error range of 10%. Meanwhile, it is found that high temperature, high pressure, high H2/oil volume ratio and removal of H2S from the exit stream of the first reactor improve the sulfur conversion in the second reactor. What is more, the influence of different process parameters on the catalyst effectiveness factor was further analyzed, the results indicate that the increase of temperature, pressure and H2/oil volume ratio can all cause the decrease of the effectiveness factor. These results contribute to a more in-depth understanding of the features and rules in the second HDS reactor, and can also give us some valuable guidance for industrial reactor simulation.









Similar content being viewed by others
Abbreviations
- DBT:
-
Dibenzothiophene
- 4,6-DMDBT:
-
4,6-Dimethyldibenzothiophene
- GC-SCD:
-
Gas chromatography with sulfur chemiluminescence detector
- UDHDS:
-
Ultra-deep hydrodesulfurization
- L–H:
-
Langmuir–Hinshelwood
- LHSV:
-
Liquid hourly space velocity
- PAHs:
-
Polycyclic aromatic hydrocarbons
- IBP:
-
Initial boiling point
- FBP:
-
Final boiling point
- HDT:
-
Hydrogenation
- \({w}_{sul}\) :
-
Mass fraction of sulfur (μg g−1)
- \(LHSV\) :
-
Liquid hourly space velocity (h−1)
- \(A\) :
-
Pre-exponential factor (mol cm−3)1−n
- \(n\) :
-
Reaction order
- \(m\) :
-
Reaction order of pressure
- β :
-
Reaction order of H2/oil volume ratio
- \({K}_{{H}_{2}S}\) :
-
Adsorption equilibrium constant of H2S component
- \(P\) :
-
Pressure (MPa)
- \({\mathrm{H}}_{2}/Oil\) :
-
H2 to oil volume ratio (v/v)
- \(R\) :
-
Universal gas constant (J mol k−1)
- \(T\) :
-
Reaction temperature (K)
- \({w}_{Sul}^{L}\) :
-
Mass fraction of total sulfur compound (μg g−1)
- \({H}_{2}S\) :
-
Hydrogen sulfide
- \({a}_{L}\) :
-
Gas–liquid interfacial area (cm−1)
- \(z\) :
-
Reactor bed length (cm)
- \({u}_{L}\) :
-
Velocity of the liquid (cm s−1)
- \({a}_{S}\) :
-
Specific surface area, liquid–solid interface (cm−1)
- \({k}_{Sul}^{S}\) :
-
Liquid–solid mass transfer coefficient for sulfur (cm s−1)
- \({c}_{Sul}^{L}\) :
-
Concentration of sulfur in the liquid phase (mol cm−3)
- \({c}_{Sul}^{S}\) :
-
Concentration of sulfur in the solid phase (mol cm−3)
- \({\rho }_{b}\) :
-
Bulk density of the catalyst particles (g cm−3)
- \({\eta }_{HDS}\) :
-
Catalyst effectiveness factor for HDS reaction
- \({r}_{Sul}\) :
-
Reaction rate for total sulfur
- \({\rho }_{1}\) :
-
Liquid density (g cm−3)
- Mw :
-
Molecular weight (g mol−1)
- \({\mu }_{L}\) :
-
Liquid viscosity (mPa s−1)
- \({G}_{L}\) :
-
Liquid mass velocity (g cm−2 s−1)
- \({D}_{sul}^{L}\) :
-
Molecular diffusivity of sulfur compound in the liquid (cm2 s−1)
- \(v\) :
-
Molar volume (cm3 mol−1)
- \({v}_{L}\) :
-
Molar volume of liquid feedstock (cm3 mol−1)
- \({T}_{meABP}\) :
-
Mean average boiling point (0R)
- NE :
-
The number of experiments
- \({A}_{c}\) :
-
Surface area (cm2)
- \(\varepsilon \) :
-
Void fraction of the catalyst bed
- \(v\) :
-
Volume (cm3)
- \(\mathrm{r}\) :
-
Particle radius (cm)
- \({d}_{L}\) :
-
Length of particle (cm)
- \({D}_{R}\) :
-
Reactor diameter (cm)
- \({d}_{s}\) :
-
Diameter of spherical catalyst particle (cm)
- \({d}_{c}\) :
-
Diameter of catalyst particle (cm)
- \({V}_{l}\) :
-
Molar gas volume of l compound at standard conditions (NL mol−1)
- \({\phi }_{Sul}\) :
-
Thiele modulus
- \({V}_{P}\) :
-
Total geometric volume of catalyst (cm−3)
- \({S}_{P}\) :
-
Total geometric external area of catalyst (cm2)
- \({K}_{Sul}\) :
-
Reaction rate coefficient of Sulfur compound
- \(De\) :
-
Effective diffusivity of sulfur in the pores of catalyst (cm2 s−1)
- \(\theta \) :
-
Particle porosity
- \(\tau \) :
-
Tortuosity factor
- \({V}_{g}\) :
-
Pore volume per unit mass of catalyst (cm3 g−1)
- \({D}_{k}\) :
-
Knudsen diffusivity (cm2 s−1)
- \({s}_{g}\) :
-
Specific surface area of particle (cm2 g−1)
- \(SSE\) :
-
Sum of square errors
- exp :
-
Experiment
- cal :
-
Calculation
- \(f\) :
-
Feedstock
- L :
-
Liquid phase
- S :
-
Solid phase
- L :
-
Liquid phase
- S :
-
Solid phase
- exp:
-
Experimental data
- cal:
-
The data calculated by the model
References
Fuel Regulations. www.dieselnet.com/standards/fuels.html
Diesel Fuel Standards and Rulemakings. https://www.epa.gov/diesel-fuel-standards/diesel-fuel-standards-and-rulemakings
Johnson TV (2010) Review of diesel emissions and control. SAE Int J Fuels Lubr 3:16–29. https://doi.org/10.4271/2010-01-0301
Albazzaz H, Marafi A, Ma X, Ansari T (2016) Hydrodesulfurization kinetics of middle distillates: a four-lumping model with consideration of nitrogen and aromatics inhibitions. Energy Fuels 31:112–121. https://doi.org/10.1021/acs.energyfuels.6b02581
Budukva S, Yeletsky P, Zaikina O et al (2019) Secondary middle distillates and their processing (review). Pet Chem 59:941–955. https://doi.org/10.1134/S0965544119090044
Nie H (2021) Key technologiest for efficient clean diesel production and commercial applocations. Pet Process Petrochem 52:103–109. https://doi.org/10.3969/j.issn.1005-2399.2021.10.018
Panzhu G (2021) Process optimization of RTS technology for ultra-Low sulfur diesel. Pet Process Petrochem Technol 23:104–111
Korsten H, Hoffmann U (1996) Three-phase reactor model of hydrotreating in pilot trickle-bed reactors. AIChE J 42:1350–1360. https://doi.org/10.1002/aic.690420515
da Novaes LR, de Resende NS, Salim VMM, Secchi AR (2017) Modeling, simulation and kinetic parameter estimation for diesel hydrotreating. Fuel 209:184–193. https://doi.org/10.1016/j.fuel.2017.07.092
Feng X, Li D, Chen J et al (2018) Kinetic parameter estimation and simulation of trickle-bed reactor for hydrodesulfurization of whole fraction low-temperature coal tar. Fuel 230:113–125. https://doi.org/10.1016/j.fuel.2018.05.023
Bej SK (2004) Revamping of diesel hydrodesulfurizers: options available and future research needs. Fuel Process Technol 85:1503–1517. https://doi.org/10.1016/j.fuproc.2003.07.002
Ho TC (2018) A theory of ultradeep hydrodesulfurization of diesel in stacked-bed reactors. AIChE J 64:595–605. https://doi.org/10.1002/aic.15969
Owusu-Boakye A, Dalai AK, Ferdous D, Adjaye J (2006) Experimental and kinetics studies of aromatic hydrogenation in a two-stage hydrotreating process using NiMo/Al2O3 and NiW/Al2O3 catalysts. Can J Chem Eng 84:572–580. https://doi.org/10.1002/cjce.5450840509
Feng X, Shen Z, Li D et al (2018) Kinetic parameter estimation and simulation of trickle-bed reactor for hydrodenitrogenation of whole-fraction low-temperature coal tar. Energy Sources 41:1–9. https://doi.org/10.1080/15567036.2018.1520360
Glii SB, Orlovi AM (2021) The influence of hydrodearomatisation reaction kinetics on the modelling of sulphur and aromatics removal from diesel fuel in an industrial hydrotreating process. Energies 14:4616. https://doi.org/10.3390/en14154616
Chen J, Mulgundmath V, Wang N (2011) Accounting for vapor−liquid equilibrium in the modeling and simulation of a commercial hydrotreating reactor. Ind Eng Chem Res 50:1571–1579. https://doi.org/10.1021/ie101550g
Macías MJ, Ancheyta J (2004) Simulation of an isothermal hydrodesulfurization small reactor with different catalyst particle shapes. Catal Today 98:243–252. https://doi.org/10.1016/j.cattod.2004.07.038
Bakhshi Ani A, Ale Ebrahim H, Azarhoosh MJ (2015) Simulation and multi-objective optimization of a trickle-bed reactor for diesel hydrotreating by a heterogeneous model using non-dominated sorting genetic algorithm II. Energy Fuels 29:3041–3051. https://doi.org/10.1021/acs.energyfuels.5b00467
Liu Y-Z, Chu G-W, Li Y-B et al (2020) Liquid-solid mass transfer in a rotating trickle-bed reactor: Mathematical modeling and experimental verification. Chem Eng Sci 220:115622. https://doi.org/10.1016/j.ces.2020.115622
Ancheyta J, Muñoz JAD, Macías MJ (2005) Experimental and theoretical determination of the particle size of hydrotreating catalysts of different shapes. Catal Today 109:120–127. https://doi.org/10.1016/j.cattod.2005.08.009
Dai M, Li S, Li Y et al (2021) Study on reaction types and environment in different regions of diesel deep hydrodesulfurization reactor. China Pet Process Petrochem Technol 023:10–20
Filho CA, Silva CM, Quadri MB, Macedo EA (2003) Tracer diffusion coefficients of citral and d-limonene in supercritical carbon dioxide. Fluid Phase Equilib 204:65–73. https://doi.org/10.1016/S0378-3812(02)00175-9
Jarullah AT, Mujtaba IM, Wood AS (2011) Kinetic model development and simulation of simultaneous hydrodenitrogenation and hydrodemetallization of crude oil in trickle bed reactor. Fuel 90:2165–2181. https://doi.org/10.1016/j.fuel.2011.01.025
Mochida I, Choi K-H (2007) Current progress in catalysts and catalysis for hydrotreating. Springer, New York, pp 257–296
Song C, Ma X (2007) Ultra-clean diesel fuels by deep desulfurization and deep dearomatization of middle distillates. In: Hsu CS, Robinson PR (eds) Practical advances in petroleum processing. Springer, New York, pp 317–372
Adam M, Calemma V, Galimberti F et al (2012) Continuum lumping kinetics of complex reactive systems. Chem Eng Sci 76:154–164. https://doi.org/10.1016/j.ces.2012.03.037
Alvarez A, Ancheyta J, Centeno G, Marroquín G (2011) A modeling study of the effect of reactor configuration on the cycle length of heavy oil fixed-bed hydroprocessing. Fuel 90:3551–3560. https://doi.org/10.1016/j.fuel.2011.03.043
Egorova M, Prins R (2004) Hydrodesulfurization of dibenzothiophene and 4,6-dimethyldibenzothiophene over sulfided NiMo/γ-Al2O3, CoMo/γ-Al2O3, and Mo/γ-Al2O3 catalysts. J Catal 225:417–427. https://doi.org/10.1016/j.jcat.2004.05.002
Jarullah AT, Mujtaba IM, Wood AS (2011) Kinetic parameter estimation and simulation of trickle-bed reactor for hydrodesulfurization of crude oil. Chem Eng Sci 66:859–871. https://doi.org/10.1016/j.ces.2010.11.016
Yin Y, Chen W, Wu G et al (2020) Kinetics towards mechanism and real operation for ultra-deep hydrodesulfurization and hydrodenitrogenation of diesel. AIChE J. https://doi.org/10.22541/au.158879111.13354748
Nguyen T, Teratani S, Tanaka R et al (2017) Development of a structure-based lumping kinetic model for light gas oil hydrodesulfurization. Energy Fuels 31:5673. https://doi.org/10.1021/acs.energyfuels.7b00360
Nguyen TTH, Teratani S, Tanaka R et al (2017) A framework for developing a structure-based lumping kinetic model for the design and simulation of refinery reactors. Comput Chem Eng 106:385–395. https://doi.org/10.1016/j.compchemeng.2017.06.025
Liu H, Li H, Zhang Y et al (2022) Asymptotic stability of Runge-Kutta method for solving nonlinear functional differential-algebraic equations. Appl Numer Math 181:277–292. https://doi.org/10.1016/j.apnum.2022.06.007
Kallinikos LE, Jess A, Papayannakos NG (2010) Kinetic study and H2S effect on refractory DBTs desulfurization in a heavy gasoil. J Catal 269:169–178. https://doi.org/10.1016/j.jcat.2009.11.005
Nguyen M-T, Pirngruber GD, Chainet F et al (2019) Molecular-level insights into coker/straight-run gas oil hydrodenitrogenation by fourier transform ion cyclotron resonance mass spectrometry. Energy Fuels 33:3034–3046. https://doi.org/10.1021/acs.energyfuels.8b04432
Funding
We thank the financial support of China Petrochemical Corporation (Sinopec Group, 120051-1) for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, Z., Ju, X., Nie, H. et al. Kinetic parameter estimation and simulation of the second reactor for hydrodesulfurization of diesel. Reac Kinet Mech Cat 136, 23–45 (2023). https://doi.org/10.1007/s11144-023-02361-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02361-8