Abstract
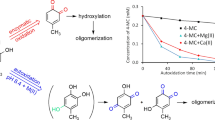
A large number of studies indicated that caffeic acid represents an important natural phenolic compound with many potential uses in medications and cosmetics, but also with therapeutic implications in cardiovascular diseases, various types of cancer, and neurological diseases. In this work we studied kinetics and reaction mechanism of caffeic acid autoxidation in weakly alkaline aqueous solutions (pH 7.4 and 8.4) in the presence of Mg(II) ions by applying high performance liquid chromatography with diode-array detection (HPLC–DAD) and electron spin resonance (ESR) spectroscopy. The results of HPLC–DAD analysis revealed that the presence of Mg(II) ions enhanced the autoxidation rate to a much greater extent than the increase in pH from 7.4 to 8.4. Analysis of DAD UV–Vis and ESR spectra, as well as comparison of the calculated logP values with retention times of chromatographically separated compounds, enabled the identification of the main initial products of caffeic acid autoxidation in the presence of Mg(II) ions as caffeic acid quinone and its dimer and indicated the nature of some minor products (hydroxylated derivatives of caffeic acid and their quinones). ESR spectroscopy disclosed that prolonged autoxidation of caffeic acid leads to the formation of polymeric, humic acid like, products. These findings may be important when considering treatments and/or storage of medications, cosmetics, and foods containing caffeic acid.
Graphical abstract









Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article and its Supplementary Information file.
References
Shahidi F, Naczk M (2004) Phenolics in food and nutraceuticals. CRC Press, Boca Raton, USA
Magnani C, Isaac VLB, Correa MA, Salgado HRN (2014) Caffeic acid: a review of its potential use in medications and cosmetics. Anal Methods 6:3203–3210. https://doi.org/10.1039/c3ay41807c
Silva H, Lopes NMF (2020) Cardiovascular effects of caffeic acid and its derivatives: a comprehensive review. Front Physiol 11:595516. https://doi.org/10.3389/fphys.2020.595516
Espíndola KMM, Ferreira RG, Narvaez LEM, Rosario ACRS, da Silva AHM, Silva AGB, Vieira APO, Monteiro MC (2019) Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front Oncol 9:541. https://doi.org/10.3389/fonc.2019.00541
Alam M, Ashraf GM, Sheikh K, Khan A, Ali S, Ansari MM, Adnan M, Pasupuleti VR, Hassan MI (2022) Potential therapeutic implications of caffeic acid in cancer signaling: past, present, and future. Front Pharmacol 13:845871. https://doi.org/10.3389/fphar.2022.845871
Alam M, Ahmed S, Elasbali AM, Adnan M, Alam S, Hassan MI, Pasupuleti VR (2022) Therapeutic implications of caffeic acid in cancer and neurological diseases. Front Oncol 12:860508. https://doi.org/10.3389/fonc.2022.860508
Cilliers JJL, Singleton VL (1989) Nonenzymic autoxidative phenolic browning reactions in caffeic acid model system. J Agric Food Chem 37:890–896. https://doi.org/10.1021/jf00088a013
Cilliers JJL, Singleton VL (1991) Characterization of the products of nonenzymic autoxidative phenolic reactions in a caffeic acid model system. J Agric Food Chem 39:1298–1303. https://doi.org/10.1021/jf00007a021
Friedman M, Jürgens HS (2000) Effect of pH on the stability of plant phenolic compounds. J Agric Food Chem 48:2101–2110. https://doi.org/10.1021/jf990489j
Maier GP, Bernt CM, Butler A (2018) Catechol oxidation: considerations in the design of wet adhesive materials. Biomater Sci 6:332–339. https://doi.org/10.1039/c7bm00884h
García P, Romero C, Brenes M, Garrido A (1996) Effect of metal cations on the chemical oxidation of olive o-diphenols in model systems. J Agric Food Chem 44:2101–2105. https://doi.org/10.1021/jf9503265
Nkhili E, Loonis M, Mihai S, El Hajji H, Dangles O (2014) Reactivity of food phenols with iron and copper ions: binding, dioxygen activation and oxidation mechanisms. Food Funct 5:1186–1202. https://doi.org/10.1039/c4fo00007b
Boilet L, Cornard JP, Lapouge C (2005) Determination of the chelating site preferentially involved in the complex of lead(II) with caffeic acid: a spectroscopic and structural study. J Phys Chem A 109:1952–1960. https://doi.org/10.1021/jp047703d
Cornard J-P, Caudron A, Merlin J-C (2006) UV-visible and synchronous fluorescence spectroscopic investigations of the complexation of Al(III) with caffeic acid, in aqueous low acidic medium. Polyhedron 25:2215–2222. https://doi.org/10.1016/j.poly.2006.01.013
De Stefano C, Foti C, Giuffré O, Sammartano S (2014) Acid-base and UV behavior of 3-(3,4-dihydroxyphenyl)-propenoic acid (caffeic acid) and complexing ability towards different divalent metal cations in aqueous solutions. J Mol Liq 195:9–16. https://doi.org/10.1016/j.molliq.2014.01.027
Arciszewska Ż, Gama S, Kalinowska M, Świderski G, Świsłocka R, Gołębiewska E, Naumowicz M, Worobiczuk M, Cudowski A, Pietryczuk A, De Stefano C, Milea D, Lewandowski W, Żyłkiewicz G (2022) Caffeic acid/Eu(III) complexes: solution equilibrium studies, structure characterization and biological activity. Int J Mol Sci 23:888. https://doi.org/10.3390/ijms23020888
Gigliuto A, Cigala RM, Irto A, Felice MR, Pettignano A, De Stefano C, Crea F (2021) The effect of metal cations on the aqueous behavior of dopamine. Thermodynamic investigation of the binary and ternary interactions with Cd2+, Cu2+ and UO2+ in NaCl at different ionic strengths and temperatures. Molecules 26:7679. https://doi.org/10.3390/molecules26247679
McBride MB, Sikora FJ (1990) Catalyzed oxidation reactions of 1,2-dihydroxybenzene (catechol) in aerated aqueous solutions of Al3+. J Inorg Biochem 39:247–262. https://doi.org/10.1016/0162-0134(90)84008-D
Lebedev AV, Ivanova MV, Timoshin AA, Ruuge EK (2007) Effect of group II metal cations on catecholate oxidation. ChemPhysChem 8:1863–1869. https://doi.org/10.1002/cphc.200700296
Živanović SC, Nikolić RS, Nikolić GM (2016) The influence of Mg(II) and Ca(II) ions on rutin autoxidation in weakly alkaline aqueous solutions. Acta Fac Med Naiss 33:163–171. https://doi.org/10.1515/afmnai-2016-0018
Nikolić GM, Živanović SC, Krstić NS, Nikolić MG (2019) The study of Mg(II) ion influence on catechol autoxidation in weakly alkaline aqueous solution. Russ J Phys Chem A 93:2656–2660. https://doi.org/10.1134/S0036024419130223
Nikolić GM, Krstić NS, Živanović SC, Nikolić GM (2022) The influence of Mg(II) and Ca(II) ions on the autoxidation of 4-methylcatechol in weakly alkaline aqueous solutions. Reac Kinet Mech Cat 135:1373–1386. https://doi.org/10.1007/s11144-022-02180-3
Živanović SC, Veselinović AM, Mitić ŽJ, Nikolić GM (2018) The study of the influence of Mg(II) and Ca(II) ions on caffeic acid autoxidation in weakly alkaline aqueous solution using MCR-ALS analysis of spectrophotometric data. New J Chem 42:6256–6263. https://doi.org/10.1039/c8nj00871j
Crichton RR (2013). In: Crichton RR, Louro RO (eds) Practical approaches to biological inorganic chemistry. Elsevier BV, Amsterdam
Monteiro CP, Matias CN, Bicho M, Santa-Clara H, Laires MJ (2016) Coordination between antioxidant defences might be partially modulated by magnesium status. Magnes Res 29:161–168. https://doi.org/10.1684/mrh.2016.0414
Lente G (2015) Deterministic kinetics in chemistry and systems biology. The dynamics of complex reaction networks. Springer, New York
Lente G (2018) Facts and alternate facts in chemical kinetics: remarks about the kinetic use of activities, termolecular processes and linearization techniques. Curr Opin Chem Eng 21:76–83. https://doi.org/10.1016/j.coche.2018.03.007
Pati S, Losito I, Palmisano F, Zambonin PG (2006) Characterization of caffeic acid enzymatic oxidation by-products by liquid chromatography coupled to electrospray ionization tandem mass spectrometry. J Chromatogr A 1102:184–192. https://doi.org/10.1016/j.chroma.2005.10.041
Antolovich M, Bedgood DR Jr, Bishop AG, Jardine D, Prenzler PD, Robards K (2004) LC-MS investigation of oxidation products of phenolic antioxidants. J Agric Food Chem 52:962–971. https://doi.org/10.1021/jf0349883
Eaton DR (1964) Complexing of metal ions with semiquinones. An electron spin resonance study. Inorg Chem 3:1268–1271. https://doi.org/10.1021/ic50019a016
Ozkorucuklu SP, Beltrán JL, Fonrodona G, Barrón D, Alsancak G, Barbosa J (2009) Determination of dissociation constants of some hydroxylated benzoic and cinnamic acids in water from mobility and spectroscopic data obtained by CE-DAD. J Chem Eng Data 54:807–811. https://doi.org/10.1021/je800595x
Haky JE, Young AM (1984) Evaluation of a simple HPLC correlation method for the estimation of the octanol-water partition coefficients of organic compounds. J Liq Chromatogr 7:675–689. https://doi.org/10.1080/01483918408073995
Mura F, Silva T, Castro C, Borges F, Zuñiga MC, Morales J, Olea-Azar C (2014) New insights in the antioxidant activity of hydroxycinnamic and hydroxybenzoic systems: spectroscopic, electrochemistry, and cellular studies. Free Rad Res 48:1473–1484. https://doi.org/10.3109/10715762.2014.965702
Ferrari RP, Laurenti E (1995) ESR spin-stabilization evidence for α-methyldopa and dopamethylester o-semiquinones obtained by peroxidasic oxidation: structural characterization and mechanistic studies. J Inorg Biochem 59:811–825. https://doi.org/10.1016/0162-0134(94)00081-K
Ferrari RP, Laurenti E, Ghibaudi EM, Gambino O (1996) ESR characterization and kinetics of the enzymatically obtained M(II)-dobutamine-o-semiquinone system. Res Chem Intermed 22:459–468. https://doi.org/10.1163/156856796X00665
Arakawa R, Yamaguchi M, Hotta H, Osaki T, Kimoto T (2004) Product analysis of caffeic acid oxidation by on-line electrochemistry/electrospray ionization mass spectrometry. J Am Soc Mass Spectrom 15:1228–1236. https://doi.org/10.1016/j.jasms.2004.05.007
Sakihama Y, Cohen MF, Grace SC, Yamasaki H (2002) Plant phenolic antioxidant and prooxidant activities: phenolics-induced oxidative damage mediated by metals in plants. Toxicology 177:67–89. https://doi.org/10.1016/S0300-483X(02)00196-8
Watanabe A, McPhail DB, Maie N, Kawasaki S, Anderson HA, Cheshire MV (2005) Electron spin resonance characteristics of humic acids from a wide range of soil types. Org Geochem 36:981–990. https://doi.org/10.1016/j.orggeochem.2005.03.002
Bolton JL, Dunlap T (2017) Formation and biological targets of quinones: cytotoxic versus cytoprotective effects. Chem Res Toxicol 30:13–37. https://doi.org/10.1021/acs.chemrestox.6b00256
Schieber A (2018) Reactions of quinones—mechanisms, structures, and prospects for food research. J Agric Food Chem 66:13051–13055. https://doi.org/10.1021/acs.jafc.8b05215
Meinhart AD, Damin FM, Calderião L, de Jesus FM, da Silva LC, da Silva CL, Filho JT, Wagner R, Godoy HT (2019) Chlorogenic and caffeic acid in 64 fruits consumed in Brazil. Food Chem 286:51–63. https://doi.org/10.1016/j.foodchem.2019.02.004
Zhang L, Li Y, Liang Y, Liang K, Zhang F, Xu T, Wang M, Song H, Liu X, Lu B (2019) Determination of phenolic acid profiles by HPLC-MS in vegetables commonly consumed in China. Food Chem 276:538–546. https://doi.org/10.1016/j.foodchem.2018.10.074
Acknowledgements
The authors would like to thank the Ministry of Education, Science and Technological Developments of Republic of Serbia (contracts No. 451-03-68/2022-14/200124 and No. 451-03-68/2022-14/200113) for financial support.
Funding
This study was supported by Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja (Grant Nos. 451-03-68/2022-14/200113, 451-03-68/2022-14/200113, 451-03-68/2022-14/200124).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nikolić, G.M., Živanović, S.C., Nikolić, M.G. et al. Kinetics and mechanism of caffeic acid autoxidation in weakly alkaline aqueous solutions in the presence of Mg(II) ions. Reac Kinet Mech Cat 136, 1169–1183 (2023). https://doi.org/10.1007/s11144-023-02358-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02358-3