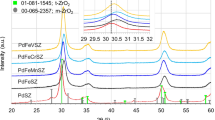
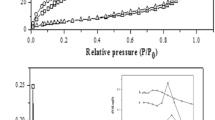
Abstract
In this study, the kinetics of the esterification of acetic acid and n-propanol catalyzed by sulfated zirconia was investigated for the first time. The effect of different temperatures (60–80 °C), n-propanol to acetic acid molar ratios (1:1–3:1), catalyst loadings (5–10 wt%), and external and internal mass transfers on the acetic acid conversions was evaluated in a batch reactor. The pseudo-homogeneous, Langmuir–Hinshelwood, and Eley–Rideal models were adjusted to the experimental data to determine the reaction mechanism. The reusability and performance of the catalyst were also reported. The results demonstrated that increasing temperature, reactants molar ratio, and catalyst loadings positively affected the formation of propyl acetate. The Eley–Rideal with acetic acid adsorbed model presented the most suitable fit, suggesting that the surface reaction at the catalyst acid sites was the limiting step in the reaction mechanism. The estimated enthalpy of the reaction indicated a slightly endothermic process. The reusability of the catalyst showed a loss of activity after successive reaction cycles. The sulfated zirconia catalyst was effective for the studied esterification reaction.







Similar content being viewed by others
Data availability
All data generated and analyzed during this study are included in this article.
References
Yadav GD, Mehta PH (1994) Heterogeneous catalysis in esterification reactions: preparation of phenethyl acetate and cyclohexyl acetate by using a variety of solid acidic catalysts. Ind Eng Chem Res 33:2198–2208. https://doi.org/10.1021/ie00033a025
Khan Z, Javed F, Shamair Z et al (2021) Current developments in esterification reaction: a review on process and parameters. J Ind Eng Chem 103:80–101. https://doi.org/10.1016/j.jiec.2021.07.018
López DE, Goodwin JG, Bruce DA, Furuta S (2008) Esterification and transesterification using modified-zirconia catalysts. Appl Catal A Gen 339:76–83. https://doi.org/10.1016/j.apcata.2008.01.009
Marx S (2016) Glycerol-free biodiesel production through transesterification: a review. Fuel Process Technol 151:139–147. https://doi.org/10.1016/j.fuproc.2016.05.033
ChemAnalyst (2021) N-Propyl Acetate Market Analysis: Plant Capacity, Production, Operating Efficiency, Technology, Demand & Supply, End-User Industries, Distribution Channel, Regional Demand. https://www.chemanalyst.com/industry-report/n-propyl-acetate-market-612. Accessed 12 Mar 2022
MarketResearch (2020) Global N-Propyl Acetate Market Research Report 2020. https://www.marketresearch.com/QYResearch-Group-v3531/Global-Propyl-Acetate-Research-13533782. Accessed 12 Mar 2022
Huang YS, Sundmacher K (2007) Kinetics study of propyl acetate synthesis reaction catalyzed by amberlyst 15. Int J Chem Kinet 39:245–253. https://doi.org/10.1002/kin.20236
Zeng D, Liu S, Gong W et al (2014) Applied catalysis A: general synthesis, characterization and acid catalysis of solid acid from peanut shell. Appl Catal A Gen 469:284–289. https://doi.org/10.1016/j.apcata.2013.09.038
Zhang B, Gao M, Geng J et al (2021) Catalytic performance and deactivation mechanism of a one-step sulfonated carbon-based solid-acid catalyst in an esterification reaction. Renew Energy 164:824–832. https://doi.org/10.1016/j.renene.2020.09.076
Chellappan S, Nair V, Sajith V, Aparna K (2018) Chinese journal of chemical engineering synthesis, optimization and characterization of biochar based catalyst from sawdust for simultaneous esterification and transesterification. Chinese J Chem Eng 26:2654–2663. https://doi.org/10.1016/j.cjche.2018.02.034
Akyalçin S, Altiokka MR (2012) Kinetics of esterification of acetic acid with 1-octanol in the presence of amberlyst 36. Appl Catal A Gen 429–430:79–84. https://doi.org/10.1016/j.apcata.2012.04.015
Wang X, Wang H, Liu Y et al (2011) A direct sulfation method for introducing the transition metal cation Co 2 + into ZrO 2 with little change in the Brønsted acid sites. J Catal 279:301–309. https://doi.org/10.1016/j.jcat.2011.01.026
Omota F, Dimian AC, Bliek A (2003) Fatty acid esterification by reactive distillation: part 2 - kinetics-based design for sulphated zirconia catalysts. Chem Eng Sci 58:3175–3185. https://doi.org/10.1016/S0009-2509(03)00154-4
Arata K (2009) Organic syntheses catalyzed by superacidic metal oxides: sulfated zirconia and related compounds. Green Chem 11:1719–1728. https://doi.org/10.1039/b822795k
Sert E, Atalay FS (2010) Kinetic study of the esterification of acetic acid with butanol catalyzed by sulfated zirconia. React Kinet Mech Catal 99:125–134. https://doi.org/10.1007/s11144-009-0117-y
Patel A, Brahmkhatri V, Singh N (2013) Biodiesel production by esterification of free fatty acid over sulfated zirconia. Renew Energy 51:227–233. https://doi.org/10.1016/j.renene.2012.09.040
Unlu D, Ilgen O, Hilmioglu ND (2016) Biodiesel additive ethyl levulinate synthesis by catalytic membrane: SO4-2/ZrO2 loaded hydroxyethyl cellulose. Chem Eng J 302:260–268. https://doi.org/10.1016/j.cej.2016.05.047
Aguzı FL, Martı L, Padro CL (2021) Esterification of succinic acid using sulfated zirconia supported on SBA-15. Chem Eng Technol. https://doi.org/10.1002/ceat.202000333
Kuwahara Y, Kaburagi W, Nemoto K, Fujitani T (2014) Esterification of levulinic acid with ethanol over sulfated Si-doped ZrO2 solid acid catalyst: study of the structure-activity relationships. Appl Catal A Gen 476:186–196. https://doi.org/10.1016/j.apcata.2014.02.032
Coker AK (2001) Modeling of chemical kinetics and reactor design. Gulf Professional Publishing, Houston
Khudsange CR (2018) Kinetics, mass transfer, and thermodynamic and statistical modeling study for esterification of valeric acid with n -butanol: homogeneous and heterogeneous catalysis. Int J Chem Kinet. https://doi.org/10.1002/kin.21195
Leyva F, Orjuela A, Miller DJ et al (2013) Kinetics of propionic acid and isoamyl alcohol liquid esterification with amberlyst 70 as catalyst. Ind Eng Chem Res. https://doi.org/10.1021/ie402349t
Sert E, Buluklu AD, Karakuş S, Atalay FS (2013) Kinetic study of catalytic esterification of acrylic acid with butanol catalyzed by different ion exchange resins. Chem Eng Process Process Intensif 73:23–28. https://doi.org/10.1016/j.cep.2013.06.004
Lee M, Wu H, Lin H (2000) Kinetics of catalytic esterification of acetic acid and amyl alcohol over dowex. Ind Eng Chem Res. https://doi.org/10.1021/ie0000764
Ali SH, Tarakmah A, Merchant SQ, Al-sahhaf T (2007) Synthesis of esters: development of the rate expression for the dowex 50 Wx8–400 catalyzed esterification of propionic acid with 1-propanol. Chem Eng Sci 62:3197–3217. https://doi.org/10.1016/j.ces.2007.03.017
Dos SPRS, Wypych F, Voll FAP et al (2016) Kinetics of ethylic esterification of lauric acid on acid activated montmorillonite (STx1-b) as catalyst. Fuel 181:600–609. https://doi.org/10.1016/j.fuel.2016.05.026
Chandane VS, Rathod AP, Wasewar KL, Sonawane SS (2017) Esterification of propionic acid with isopropyl alcohol over ion exchange resins : optimization and kinetics. Korean J Chem Eng 34:249–258. https://doi.org/10.1007/s11814-016-0249-5
Yan GX, Wang A, Wachs IE, Baltrusaitis J (2019) Applied catalysis A, general critical review on the active site structure of sulfated zirconia catalysts and prospects in fuel production. Appl Catal A Gen 572:210–225. https://doi.org/10.1016/j.apcata.2018.12.012
Osatiashtiani A, Durndell LJ, Manayil JC et al (2016) Influence of alkyl chain length on sulfated zirconia catalysed batch and continuous esterification of carboxylic acids by light alcohols. Green Chem 18:5529–5535. https://doi.org/10.1039/c6gc01089j
Silva VR, Hamerski F, Weschenfelder TA et al (2016) Equilibrium, kinetic, and thermodynamic studies on the biosorption of Bordeaux S dye by sericin powder derived from cocoons of the silkworm Bombyx mori. Desalin Water Treat 57:5119–5129. https://doi.org/10.1080/19443994.2014.996776
Hamerski F, Dusi GG, Fernandes dos Santos JT et al (2020) Esterification reaction kinetics of acetic acid and n-pentanol catalyzed by sulfated zirconia. Int J Chem Kinet 52:499–512. https://doi.org/10.1002/kin.21365
Lente G (2018) Facts and alternative facts in chemical kinetics: remarks about the kinetic use of activities, termolecular processes, and linearization techniques. Curr Opin Chem Eng 21:76–83. https://doi.org/10.1016/j.coche.2018.03.007
Fogler HS (2006) Elements of chemical reaction engineering, 4th edn. Prentice-Hall, New York
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Automat Contr 19:716–723. https://doi.org/10.1109/TAC.1974.1100705
Burnham KP, Anderson DR (2004) Multimodel inference: understanding AIC and BIC in model selection. Sociol Methods Res 33:261–304. https://doi.org/10.1177/0049124104268644
Perry RH, Green DW (2006) Perry’s chemical engineers’ handbook, 7th edn. McGraw-Hill, New York
Wilke CR, Chang P (1955) Correlation of diffusion coefficients in dilute solutions. AIChE J 1:264–270. https://doi.org/10.1002/aic.690010222
Jyoti G, Keshav A, Anandkumar J (2016) Experimental and kinetic study of esterification of acrylic acid with ethanol using homogeneous catalyst. Int J Chem React Eng. https://doi.org/10.1515/ijcre-2015-0131
Bhusari AA, Mazumdar B, Rathod AP, Khonde RD (2020) Kinetics of catalyzed esterification of acetic acid with n-butanol using carbonized agro-waste. Int J Chem Kinet 52:450–462. https://doi.org/10.1002/kin.21361
Ju IB, Lim HW, Jeon W et al (2011) Kinetic study of catalytic esterification of butyric acid and n-butanol over Dowex 50Wx8-400. Chem Eng J 168:293–302. https://doi.org/10.1016/j.cej.2010.12.086
Saravanan K, Tyagi B, Bajaj HC (2016) Nano-crystalline, mesoporous aerogel sulfated zirconia as an efficient catalyst for esterification of stearic acid with methanol. Appl Catal B Environ 192:161–170. https://doi.org/10.1016/j.apcatb.2016.03.037
Liu W, Tan C (2001) Liquid-phase esterification of propionic acid with n-Butanol kinetics, catalysis, and reaction engineering liquid-phase esterification of propionic acid with n-Butanol. Ind Eng Chem Res 40:3281–3286
Zeng Z, Cui L, Xue W et al (2012) Recent developments on the mechanism and kinetics of esterification reaction promoted by various catalysts. Chem Kinet. https://doi.org/10.5772/38106
Acknowledgements
The authors thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico—Brasil (CNPq) (Grant/award number: 1688/2018)—for the financial support and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001 for the scholarship support.
Funding
This work was supported by the Coordination for the Improvement of Higher Education Personnel (CAPES) and the National Council for Scientific and Technological Development (CNPq) (Grant/Award Numbers: 1688/2018).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by GSM, VFB, GGD, LHPD, FH, and VRdS. The first draft of the manuscript was written by VRdS and GSM and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marques, G.S., Domingues, L.H.P., Fitz Bregenski, V. et al. Synthesis of propyl acetate by sulfated zirconia: kinetics and mechanism modeling. Reac Kinet Mech Cat 136, 397–414 (2023). https://doi.org/10.1007/s11144-023-02353-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02353-8