Abstract
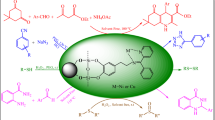
New copper hydrazone complexes [CuL1(OAc)](OAc), and [CuL2(OAc)], (L1and HL2 = Schiff base of benzhydrazide with 2-pyridine and 2-hydroxy 3-methoxy benzaldehyde, respectively) were synthesized. The heterogeneous nanocatalysts were obtained through immobilization of homogenous catalyst on magnetic nanoparticles (Fe3O4@SiO2@CPTMS). Characterization of heterogeneous nanocatalysts was performed by different techniques including FTIR, XRD and SEM. The catalytic behavior of homogenous and heterogeneous nanocatalysts were evaluated for oxidation of olefins. Comparing the catalytic behavior of homogenous and heterogeneous confirm that heterogeneous catalysts were more efficient than homogenous catalysts. Based on results, the function of Fe3O4@SiO2@CPTMS/[CuL1(OAc)](OAc) and Fe3O4@SiO2@CPTMS/[CuL2(OAc)] was notable for oxidation of styrene with 98%, 93% conversion and 81%, 68% selectivity towards benzaldehyde, respectively. By coupling Fe3O4 with hydrazone complexes not only can separate the catalysts from reaction medium easily, but also achieve to higher recyclability (up to four cycles). Moreover, to further assist the experimental studies, a comprehensive theoretical calculations are also performed by using density functional theory (DFT) calculations.












Similar content being viewed by others
Data availability
All data are available on request.
References
Plass W (2003) Supramolecular interactions of vanadate species: vanadium(V) complexes with N-salicylidenehydrazides as versatile models. Coord Chem Rev 237:205–212. https://doi.org/10.1016/S0010-8545(02)00228-x
Nica S, Pohlmann A, Plass W (2005) Vanadium(V) oxoperoxo complexes with side chain substituted N-salicylidenehydrazides: modeling supramolecular interactions in vanadium haloperoxidases. Eur J Inorg Chem 5:2032–2036. https://doi.org/10.1002/ejic.200401060
Plass W, Pohlmann A, Yozgatli H (2000) Short communication N-salicylidenehydrazides as versatile tridentate ligands for dioxovanadium(V) complexes. J Inorg Biochem 80:181–183. https://doi.org/10.1016/s0162-0134(00)00029-5
Yunpeng JD, Wei Ch, Fu X (2016) Synthesis and biological evaluation of heterocyclic hydrazone transition metal complexes as potential anticancer agents Yunpeng. RSC Adv 6:109718–197725. https://doi.org/10.1039/C6RA23477A
Hu S, Song J, Zhao F, Meng X (2015) Highly sensitive and selective colorimetric naked-eye detection of Cu2+ in aqueous medium using a hydrazone chemosensor. Sens Actuators B 215:241–248. https://doi.org/10.1016/j.snb.2015.03.059
Ward MD (2005) Near-infrared electrochromic materials for optical attenuation based on transition-metal coordination complexes. J Solid State Electrochem 9:778–787. https://doi.org/10.1007/s10008-005-0668-4
Anwar MU, Shuvaev KV, Dawe LN, Thompson LK (2011) Polynuclear Fen complexes (n = 1, 2, 4, 5) of polytopic hydrazone ligands with Fe(II), Fe(III) and mixed oxidation state combinations polynuclear Fe. Inorg Chem 50(23):12141–12154. https://doi.org/10.1021/ic201891h
Ghorbanloo M, Bikas R, Jafari S, Krawczyk MS (2018) Synthesis, structural characterization and catalytic potential of oxidovanadium(IV) and dioxidovanadium(V) complexes with thiazole-derived NNN-donor ligand. J Coord Chem 8972:1–16. https://doi.org/10.1080/00958972.2018.1465941
Hosseini-monfared H, Bikas R, Mahboubi-anarjan P, Blake AJ, Lippolis V, Arslan NB, Kazak C (2014) Oxidovanadium(V) complexes containing hydrazone based O, N, O-donor ligands: synthesis, structure, catalytic properties and theoretical calculations. Polyhedron 69:90–102. https://doi.org/10.1016/j.poly.2013.11.020
Kumari S, Das B, Ray S (2019) An insight into the catalytic activity of palladium Schiff-base complexes towards the Heck coupling. Dalton Trans 4:15942–15954. https://doi.org/10.1039/c9dt03341f
Naeimi H, Moradian M (2008) Alumina-supported metal(II) Schiff base complexes as heterogeneous catalysts in the high-regioselective cleavage of epoxides to halohydrins by using elemental halogen. Polyhedron 27:3639–3645. https://doi.org/10.1016/j.poly.2008.08.015
Gupta KC, Kumar A, Lin C (2009) Polymer-supported Schiff base complexes in oxidation reactions. Coord Chem Rev 253:1926–1946. https://doi.org/10.1016/j.ccr.2009.03.019
Gawande MB, Rathi AK, Nogueira ID, Varma RS, Branco PS (2013) Green chemistry. Green Chem 15:1895–1899. https://doi.org/10.1039/c3gc40457a
Masteri-farahani M, Adegozali S, Matin A (2015) Hybrid organometallic-inorganic nanomaterial: acetyl ferrocene Schiff base immobilized on silica coated magnetite nanoparticles abstract. J Nanostruct 5:337–344. https://doi.org/10.7508/JNS.2015.04.003
Xinzhe Li RL, Fang Y, Hu Y, Huo H, Zhao SH, Long X, Ma J (2015) Mesoporous titanium dioxide coating on gold modified silica nanotubes: a tube-in-tube titanium nanostructure for visible-light photocatalysts. RSC Adv 5:62962–62969. https://doi.org/10.1039/C5RA11934K
Gawande MB, Monga Y, Zboril R, Sharma RK (2015) Silica-decorated magnetic nanocomposites for catalytic applications. Coord Chem Rev 288:118–143. https://doi.org/10.1016/j.ccr.2015.01.001
Ghorbani-choghamarani A, Darvishnejad Z, Tahmasbi B (2015) Schiff base complexes of Ni Co, Cr, Cd and Zn supported on magnetic. Inorg Chim Acta 435:223–231. https://doi.org/10.1016/j.ica.2015.07.004
Eftekhari-sis B, Akbari M, Amini M, Ashouri F (2016) Oxoperoxo tungsten(VI) complex immobilized on Schiff base-modified Fe3O4 magnetic nanoparticles as a heterogeneous catalyst for oxidation of alcohols with hydrogen peroxide. J Coord Chem 8972:1–10. https://doi.org/10.1080/00958972.2016.1251588
Rayati S, Khodaei E, Jafarian M, Wojtczak A (2017) Mn-Schiff base complex supported on magnetic nanoparticles: synthesis, crystal structure, electrochemical properties and catalytic activities for oxidation of olefins and sulfides. Polyhedron 133:327–335. https://doi.org/10.1016/j.poly.2017.05.049
Tavakoli M, Samira H, Mehdi S, Ardakani H, Pakdin Z (2018) A manganese Schiff base complex immobilized on copper–ferrite magnetic nanoparticles as an efficient and recyclable nanocatalyst for selective oxidation of alcohols. Transit Met Chem 43:579–589. https://doi.org/10.1007/s11243-018-0244-2
Veisi H, Rashtiani A, Rostami A, Shirinbayan M, Hemmati S (2019) Chemo-selective oxidation of sulfide to sulfoxides with H2O2 catalyzed by oxo-vanadium/Schiff-base complex immobilized on modified magnetic Fe3O4 nanoparticles as a heterogeneous and recyclable nanocatalyst. Polyhedron 157:358–366. https://doi.org/10.1016/j.poly.2018.09.034
Maleki A, Taheri-ledari R, Ghalavand R, Firouzi-haji R (2020) Palladium-decorated o-phenylenediamine-functionalized Fe3O4/SiO2 magnetic nanoparticles: a promising solid-state catalytic system used for Suzuki–Miyaura coupling reactions. J Phys Chem Solids 136:109200. https://doi.org/10.1016/j.jpcs.2019.109200
Al-Qahtani SD, Alsoliemy A, Almehmadi SJ, Alkhamis KH, Alrefaei AF, Zaky R, El-Metwaly N (2021) Green synthesis for new Co(II), Ni(II), Cu(II) and Cd(II) hydrazone-based complexes; characterization, biological activity and electrical conductance of nano-sized copper sulphate. J Mol Struct 1244:131238–131250. https://doi.org/10.1016/j.molstruc.2021.131238
Adam MSS, Khalil A (2022) Nickel(II), copper(II), and vanadyl(II) complexes with tridentate nicotinoyl hydrazone derivative functionalized as effective catalysts for epoxidation processes and as biological reagents. J Taiwan Inst Chem Eng 132:104192–104202. https://doi.org/10.1016/j.jtice.2021.104192
Thompson KH, Orvig C (2003) Boon and bane of metal ions in medicine. Science 300:936–939. https://doi.org/10.1126/science.1083004
Liu X, Ma Z, Xing J, Liu H (2004) Preparation and characterization of amino-silane modified superparamagnetic silica nanospheres. J Magn Magn Mater 270:1–6. https://doi.org/10.1016/j.jmmm.2003.07.006
Fe N (2014) Nano Fe3O4 supported biimidazole Cu(I) complex. RSC Adv 4:23116–23124. https://doi.org/10.1039/c4ra03333g
Mirzazadeh H, Lashanizadegan M (2018) Improving the catalytic activity of magnetic Fe3O4/ZnO–CdO/reduced graphene oxide for ultrasonic degradation of the organic pollutants and the green oxidation of olefins. Solid State Sci 79:48–57. https://doi.org/10.1016/j.solidstatesciences.2018.03.010
Lashanizadegan M, Mirzazadeh H, Esfandiari Z (2019) Evaluation performance of Fe–Mn–Ce–O mixed metal oxides and Fe–Mn–Ce–O/montmorillonite for adsorption of azo dyes in aqueous solution and oxidation reaction. Mater Res Express 6:125028–125044. https://doi.org/10.1088/2053-1591/ab5550
Lashanizadegan M, Anafcheh M, Mirzazadeh H, Gholipoor P (2020) Efficient Cd/Ce nanoparticles supported on reduced graphene oxide for the reduction of 4-nitrophenol and the oxidation of olefins: experimental and theoretical study. Mater Res Bull 125:110773–110784. https://doi.org/10.1016/j.materresbull.2020.110773
Lashanizadegan M, Nikoofar K, Aghaei A, Mehrikaram F, Mirzazadeh H (2019) Immobilized Cu(II) and Co(II) Schiff base complexes on the surface of functionalized magnetized multiwalled carbon nanotubes: novel catalysts for oxidation and solvent-free pseudo six-component condensation reaction. Solid State Sci 95:105937–105948. https://doi.org/10.1016/j.solidstatesciences.2019.105937
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104–154115. https://doi.org/10.1063/1.3382344
Grimme S, Ehrlich S, Goerigk L (2011) Effect of the damping function in dispersion corrected density functional theory. J Comput Chem 32:1456–1465. https://doi.org/10.1002/jcc.21759
Boys SF, Bernardi FD (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19(4):553–566. https://doi.org/10.1080/00268977000101561
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3094. https://doi.org/10.1021/cr9904009
Tomasi J, Persico M (1994) Molecular interactions in solution: an overview of methods based on continuous distributions of the solvent. Chem Rev 94:2027–2094. https://doi.org/10.1021/cr00031a013
Ghiasi M, Gholami S (2020) Quantum mechanical study of human carbonic anhydrase II incomplex with polyamines as novel inhibitors: kinetic and thermodynamic investigation. Comput Theor Chem 1186:112911. https://doi.org/10.1016/j.comptc.2020.112911
Ghiasi M, Bavafa S, Zahedi M (2021) QM study of interaction between arginine amino acid and Au clusters and the effects on arginine acidity. Gold Bull. https://doi.org/10.1007/s13404-021-00292-7
Reed AE, Carpenter JE, Weinhold F, Glendening ED (1992) What is NBO analysis and how is it useful? Int Rev Phys Chem. https://doi.org/10.1080/0144235X.2016.1192262
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE et al (2009) Gaussian 09, revision A.08. Gaussian Inc, Wallingford, pp 5648–5652
Salehzadeh S, Golbedaghi R, Tidmarsh IS, Al-rasbi NK, Adams H, Ward MD (2008) Cd(II) and Mn(II) complexes of a new hexadentate Schiff base ligand derived from an asymmetric tripodal tetraamine and 2-pyridinecarboxaldehyde. Polyhedron 27:3549–3556. https://doi.org/10.1016/j.poly.2008.08.019
Abd-elzaher MM (2001) Spectroscopic characterization of some tetradentate Schiff bases and their complexes with nickel, copper and zinc. J Chin Chem Soc Chem Soc 48:153–158. https://doi.org/10.1002/jccs.200100027
Yeap G, Ha S, Ishizawa N, Suda K (2003) Synthesis, crystal structure and spectroscopic study of para substituted 2-hydroxy-3-methoxybenzalideneanilines. J Mol Struct 658:87–99. https://doi.org/10.1016/S0022-2860(03)00453-8
Hayvali Z, Dal H (2011) Syntheses, characterizations and structures of NO donor Schiff base ligands and nickel(II) and copper(II) complexes. J Mol Struct 997:53–59. https://doi.org/10.1016/j.molstruc.2011.04.037
Cancino HGP, Vega A, Santiago-Portillo A, Navalon S, Alvaro M, Aguirre P, Spodine E (2016) Novel copper(II)–lanthanum(III) metal organic framework as selective catalyst for the aerobic oxidation of benzylic hydrocarbons and cycloalkenes. Catal Sci Technol 6:3727–3736. https://doi.org/10.1039/C5CY01448D
Farzaneh F, Taghavi J, Malakooti R, Ghandi M (2006) Immobilized vitamin B12 within nanoreactors of MCM-41 as selective catalyst for oxidation of organic substrates. J Mol Catal A 244:252–257. https://doi.org/10.1016/j.molcata.2005.08.058
Slaughter LM, Collman JP, Eberspacher TA et al (2004) Radical autoxidation and autogenous O2 evolution in manganese−porphyrin catalyzed alkane oxidations with chlorite. Inorg Chem 43:6641–6647. https://doi.org/10.1021/ic049922j
Neuenschwander U, Hermans I (2011) The conformations of cyclooctene: consequences for epoxidation chemistry. J Org Chem 76:10236–10240. https://doi.org/10.1021/jo202176j
Islam SM, Mondal P, Mukherjee S (2011) A reusable polymer anchored copper(II) complex catalyst for the efficient oxidation of olefins and aromatic alcohol. Polym Adv Technol 22:933–941. https://doi.org/10.1002/pat.1598
Zhu X, Shen R, Zhang L (2014) Catalytic oxidation of styrene to benzaldehyde over a copper Schiff-base/SBA-15 catalyst. Chin J Catal 35:1716–1726. https://doi.org/10.1016/S1872-2067(14)60131-5
Yang Y, Zhang Y, Hao S, Kan Q (2011) Tethering of Cu(II), Co(II) and Fe(III) tetrahydro-salen and salen complexes onto amino-functionalized SBA-15: effects of salen ligand hydrogenation on catalytic performances for aerobic epoxidation of styrene. Chem Eng J 171:1356–1366. https://doi.org/10.1016/j.cej.2011.05.047
Anand N, Hari K, Reddy P, Swapna V, Seetha K, Rao R, Burri DR (2011) Fe(III) complex anchored SBA-15 is a new heterogeneous catalyst for the cleavage of aliphatic C@C bond of styrene and its derivatives. Microporous Mesoporous Mater 143:132–140. https://doi.org/10.1016/j.micromeso.2011.02.017
Mohan N, Suresh CH (2014) A molecular electrostatic potential analysis of hydrogen, halogen, and dihydrogen bonds. J Phys Chem A 118:1697–1705. https://doi.org/10.1021/jp4115699
Acknowledgements
The authors acknowledged the research council of Alzahra University for their financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lashanizadegan, M., Habibi, N., Mirzazadeh, H. et al. Immobilized magnetic copper hydrazone complexes for oxidation of styrene to benzaldehyde by tert-butylhydroxyperoxide: an experimental and theoretical approach. Reac Kinet Mech Cat 135, 3223–3242 (2022). https://doi.org/10.1007/s11144-022-02304-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-022-02304-9