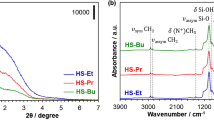
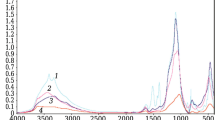
Abstract
CTA-MCM-41 hybrid silica was prepared using a 40 °C non hydrothermal method for 2 h and was characterized using X-ray diffraction, scanning electron microscopy, transmission electron microscopy and thermogravimetric analysis. Using these techniques was possible to confirm the structural organization with a highly organized hexagonal matrix of the MCM-41 type, quantify the presence of hexadecyltrimethylammonium cations present in the pores of silica and, consequently, to measure the concentration of the catalytic sites present in the material, 1.83 mmol g−1. This catalyst was used in the transesterification of the esters with the aim of determining the influence of the length of the ester side-chain on the reaction kinetics. The ethyl esters tested had the length of the ester side-chain in the range 1–4 carbons. The catalytic tests were performed at temperatures ranging from 20 to 50 °C, employing a methanol/ester molar ratio of 6:1 and 4% of catalyst relative to the total reactants mass. Conversion close to 80% was observed for the ethyl acetate at 40 °C and decreased as the length of the ester side-chain increased. Fitting using a pseudo-homogeneous reversible first order model enabled determination of the kinetic parameters for each reaction with activation energies between 41.3 and 48.3 kJ mol−1. Inductive and diffusional effects explain the slower reaction rate and higher activation energy as the size of the molecule increase.






Similar content being viewed by others
References
Xu W, Gao L, Wang S, Xiao G (2014) Biodiesel production in a membrane reactor using MCM-41 supported solid acid catalyst. Bioresour Technol 159:286–291. https://doi.org/10.1016/j.biortech.2014.03.004
Chua SY, Periasamy LA, Goh CMH et al (2019) Biodiesel synthesis using natural solid catalyst derived from biomass waste: a review. J Ind Eng Chem 80:1–78. https://doi.org/10.1016/j.jiec.2019.09.022
Gómez JM, Romero MD, Callejo V (2013) Heterogeneous basic catalysis for upgrading of biofuels. Catal Today 218:143–147. https://doi.org/10.1016/j.cattod.2013.04.027
Lima AL, Ronconi CM, Mota CJA (2016) Heterogeneous basic catalysts for biodiesel production. Catal Sci Technol 6:2877–2891. https://doi.org/10.1039/C5CY01989C
Martins L, Bonagamba TJ, de Azevedo ER et al (2006) Surfactant containing Si-MCM-41: An efficient basic catalyst for the Knoevenagel condensation. Appl Catal A Gen 312:77–85. https://doi.org/10.1016/j.apcata.2006.06.035
Kubota Y, Nishizaki Y, Ikeya H et al (2004) Organic—silicate hybrid catalysts based on various defined structures for Knoevenagel condensation. Microporous Mesoporous Mater 70:135–149
Alkimim IP, Silva LL, Cardoso D (2017) Synthesis of hybrid spherical silicas and application in catalytic transesterification reaction. Microporous Mesoporous Mater 254:37–44. https://doi.org/10.1016/j.micromeso.2017.04.018
Dossin TF, Reyniers MF, Marin GB (2006) Kinetics of heterogeneously MgO-catalyzed transesterification. Appl Catal B 62:35–45. https://doi.org/10.1016/j.apcatb.2005.04.005
Peng Y, Cui X, Zhang Y et al (2013) Kinetic study of transesterification of methyl acetate with ethanol catalyzed by 4-(3-methyl-1-imidazolio)-1-butanesulfonic acid triflate. Appl Catal A Gen 466:131–136. https://doi.org/10.1016/j.apcata.2013.06.048
Darnoko D, Cheryan M (2000) Kinetics of palm oil transesterification in a batch reactor. JAOCS 77:1263–1267. https://doi.org/10.1007/s11746-000-0198-y
Reyero I, Arzamendi G, Zabala S, Gandía LM (2015) Kinetics of the NaOH-catalyzed transesterification of sunflower oil with ethanol to produce biodiesel. Fuel Process Technol 129:147–155. https://doi.org/10.1016/j.fuproc.2014.09.008
Van de Steene E, De Clercq J, Thybaut JW (2014) Ion-exchange resin catalyzed transesterification of ethyl acetate with methanol: Gel versus macroporous resins. Chem Eng J 242:170–179. https://doi.org/10.1016/j.cej.2013.12.025
Ali SH, Al-Rashed O, Azeez FA, Merchant SQ (2011) Potential biofuel additive from renewable sources - Kinetic study of formation of butyl acetate by heterogeneously catalyzed transesterification of ethyl acetate with butanol. Bioresour Technol 102:10094–10103. https://doi.org/10.1016/j.biortech.2011.08.033
Xu B, Zhang W, Xuemei Z, Zhou C (2008) Kinetic study of transesterification of methyl acetate with n-butanol catalyzed by NKC-9. Int J Chem Kinet 43:101–106. https://doi.org/10.1002/kin
Araújo JA, Cruz FT, Cruz IH, Cardoso D (2013) Encapsulation of polymers in CTA-MCM-41 via microemulsion. Microporous Mesoporous Mater 180:14–21. https://doi.org/10.1016/j.micromeso.2013.05.010
Berrios M, Siles J, Martı MA, Martı A (2007) A kinetic study of the esterification of free fatty acids (FFA) in sunflower oil. Fuel 86:2383–2388. https://doi.org/10.1016/j.fuel.2007.02.002
Kumar D, Schumacher K, Von HCF et al (2001) MCM-41, MCM-48 and related mesoporous adsorbents : their synthesis and characterisation. Colloids Surfaces A 188:109–116
Selvam P, Bhatia SK, Sonwane CG (2001) Recent advances in processing and characterization of periodic mesoporous MCM-41 silicate molecular sieves. Ind Eng Chem Res 40:3237–3261. https://doi.org/10.1021/ie0010666
Meynen V, Cool P, Vansant EF (2009) Verified syntheses of mesoporous materials. Microporous Mesoporous Mater 125:170–223. https://doi.org/10.1016/j.micromeso.2009.03.046
Meléndez-Ortiz HI, Mercado-silva A, García-cerda LA et al (2013) Hydrothermal Synthesis of Mesoporous Silica MCM-41 Using Commercial Sodium Silicate. J Mex Chem Soc 57:73–79
Zhao XS, Lu GQ, Whittaker AK et al (1997) Comprehensive study of surface chemistry of MCM-41 using 29Si CP/MAS NMR, FTIR, pyridine-TPD, and TGA. J Phys Chem B 101:6525–6531. https://doi.org/10.1021/jp971366+
Hayduk W, Laudie H (1974) Prediction of diffusion coefficients for nonelectrolytes in dilute aqueous solutions. AIChE J 20:611–615. https://doi.org/10.1002/aic.690200329
Alonso DM, Granados ML, Mariscal R, Douhal A (2009) Polarity of the acid chain of esters and transesterification activity of acid catalysts. J Catal 262:18–26. https://doi.org/10.1016/j.jcat.2008.11.026
Liu Y, Lotero E, Goodwin JG (2006) Effect of carbon chain length on esterification of carboxylic acids with methanol using acid catalysis. J Catal 243:221–228. https://doi.org/10.1016/j.jcat.2006.07.013
Bozek-Winkler E, Gmehling J (2006) Transesterification of methyl acetate and n-butanol catalyzed by Amberlyst 15. Ind Eng Chem Res 45:6648–6654. https://doi.org/10.1021/ie060536e
Acknowledgements
The authors are grateful for the financial support provided by the following Brazilian agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Grant #132824/2018-3 and Grant #141307/2018-8) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, #001). The authors thank the Structural Characterization Laboratory of UFSCar (LCE/DEMa/UFSCar) for the microscopy analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Paula, L.N.R., de Paula, G.M. & Cardoso, D. Kinetic study of ethyl ester transesterification using a hybrid silica catalyst. Reac Kinet Mech Cat 135, 2427–2439 (2022). https://doi.org/10.1007/s11144-022-02258-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-022-02258-y