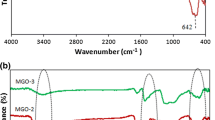
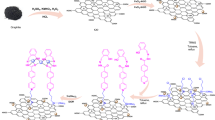
Abstract
In this study, hydantoin derivative 5-methyl-5-(4-pyridyl)hydantoin (HL) was covalently functionalized onto graphene oxide/magnetic graphene oxide nanosheets. The chemical structure of the functionalized graphene oxide nanosheets and the HL were characterized with FT-IR, XRD, EDS, TEM, UV–Vis, FE-SEM, 1H, and 13C NMR spectra. We demonstrate that the (GO/HL-Pd) and (NPs@GO/HL-Pd) compounds can act as efficient nano-catalyst for the Suzuki–Miyaura reaction under aqueous and aerobic conditions in a short time. The effect of the concentration of the variable such as temperature, time, base, and nano-catalysts value on the performance of the Suzuki–Miyaura reaction was evaluated using statistical methods (DoE) for both synthesized nanocatalysts in a systematic sequential study. According to the results, the magnetized compound with iron nanoparticles has a higher catalytic activity. Therefore, the optimum point of the design involves a 3.3 ml solvent, 0.0175 mol% of NPs@GO/HL-Pd catalyst, 2.75 eq K2CO3, furthermore a 3.5 h reaction time, and reaction temperature of 77.5 °C. Optimal conditions lead to the maximum yield value. Furthermore, the as-prepared nano-catalysts can be easily recovered and reused after a catalysis reaction.




Similar content being viewed by others
References
Chen JH, Jang C, Xiao S, Ishigami M, Fuhrer MS (2008) Intrinsic and extrinsic performance limits of graphene devices on SiO2. Nat Nanotechnol 3:206
Geim AK, Kim P et al (2008) Graphene on gallium arsenide: engineering the visibility. Sci Am 298:68–75
Dong W, Cheng S, Feng C et al (2017) Fabrication of highly dispersed Pd nanoparticles supported on reduced graphene oxide for catalytic reduction of 4-nitrophenol. Catal Commun 90:70–74
Li B, Cao H, Shao J et al (2011) Co3O4@graphene composites as anode materials for high-performance lithium ion batteries. Inorg Chem 50:1628–1632
Georgakilas V, Bourlinos AB, Zboril R et al (2010) Organic functionalisation of graphenes. Chem Commun 46:1766–1768
Kerscher B, Appel A-K, Thomann R, Mülhaupt R (2013) Treelike polymeric ionic liquids grafted onto graphene nanosheets. Macromolecules 46:4395–4402
Shang N, Gao S, Feng C, Zhang H, Wang C, Wang Z (2013) Graphene oxide supported N-heterocyclic carbene-palladium as a novel catalyst for the Suzuki–Miyaura reaction. RSC Adv 3(44):21863–21868
Meusel M, Gütschow M (2004) Recent developments in hydantoin chemistry. A review. Org Prep Proced Int 36:391–443
Sabine TM, Hogg S (1969) Acta Crystallogr. Sect B: Struct Crystallogr Cryst Chem
Cannas M, Carta G, Marongiu G, Trogu EF (1974) The crystal structure of thiazolidine-2-thionepentacarbonyltungsten. Acta Crystallogr Sect B Struct Crystallogr Cryst Chem 30:2252–2254
Safari J, Javadian L (2013) A one-pot synthesis of 5,5-disubstituted hydantoin derivatives using magnetic Fe3O4 nanoparticles as a reusable heterogeneous catalyst. C R Chim 16:1165–1171
Kovtyukhova NI, Karpenko GA, Chuiko AA (1992) Complex formation by transition metal ions in aqueous suspensions of graphite oxide. Russ J Inorg Chem 37:566–569
Mastalir Á, Király Z, Patzko A et al (2008) Synthesis and catalytic application of Pd nanoparticles in graphite oxide. Carbon N Y 46:1631–1637
Sabater S, Mata JA, Peris E (2014) Catalyst enhancement and recyclability by immobilization of metal complexes onto graphene surface by noncovalent interactions. ACS Catal 4:2038–2047
Park JH, Raza F, Jeon S-J et al (2014) Recyclable N-heterocyclic carbene/palladium catalyst on graphene oxide for the aqueous-phase Suzuki reaction. Tetrahedron Lett 55:3426–3430
Hoseini SJ, Heidari V, Nasrabadi H (2015) Magnetic Pd/Fe3O4/reduced-graphene oxide nanohybrid as an efficient and recoverable catalyst for Suzuki–Miyaura coupling reaction in water. J Mol Catal A 396:90–95
Kantam ML, Srinivas P, Yadav J et al (2009) Trifunctional N,N,O-terdentate amido/pyridyl carboxylate ligated Pd(II) complexes for Heck and Suzuki reactions. J Org Chem 74:4882–4885
Deagostino A, Prandi C, Tabasso S, Venturello P (2010) The Heck reaction applied to 1,3- and 1,2-unsaturated derivatives, a way towards molecular complexity. Molecules 15:2667–2685
Scheuermann GM, Rumi L, Steurer P et al (2009) Palladium nanoparticles on graphite oxide and its functionalized graphene derivatives as highly active catalysts for the Suzuki–Miyaura coupling reaction. J Am Chem Soc 131:8262–8270
Ha H-W, Kim IY, Hwang S-J, Ruoff RS (2011) One-pot synthesis of platinum nanoparticles embedded on reduced graphene oxide for oxygen reduction in methanol fuel cells. Electrochem Solid State Lett 14:B70
Lipshutz BH, Taft BR, Abela AR et al (2012) Catalysis in the service of green chemistry: nobel prize-winning palladium-catalysed cross-couplings, run in water at room temperature: Heck, Suzuki–Miyaura and Negishi reactions carried out in the absence of organic solvents, enabled by micellar catalysis. Platin Met Rev 56:62
Deng Y, Deng C, Yang D et al (2005) Preparation, characterization and application of magnetic silica nanoparticle functionalized multi-walled carbon nanotubes. Chem Commun. https://doi.org/10.1039/b511683j
Moniriyan F, Sabounchei SJ (2021) A comparative study of catalytic activity on iron-based carbon nanostructured catalysts with Pd loading: using the Box–Behnken design (BBD) method in the Suzuki–Miyaura coupling. Appl Organomet Chem 35:1–20. https://doi.org/10.1002/aoc.6415
Dejaegher B, Vander Heyden Y (2011) Experimental designs and their recent advances in set-up, data interpretation, and analytical applications. J Pharm Biomed Anal 56:141–158
Murray PM, Tyler SNG, Moseley JD (2013) Beyond the numbers: charting chemical reaction space. Org Process Res Dev 17:40–46
Tye H (2004) Application of statistical ‘design of experiments’ methods in drug discovery. Drug Discov Today 9:485–491
Leardi R (2009) Experimental design in chemistry: a tutorial. Anal Chim Acta 652:161–172
Murray PM, Bellany F, Benhamou L, Bučar DK, Tabor AB, Sheppard TD (2016) The application of design of experiments (DoE) reaction optimisation and solvent selection in the development of new synthetic chemistry. Org Biomol Chem 14:2373
Eknoian MW, Webb TR, Worley SD et al (1999) 5-(4-Pyridyl)-5-phenylhydantoin. Acta Crystallogr Sect C Cryst Struct Commun 55:405–407
Hummers WS Jr, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Gao W (2015) Graphene oxide: reduction recipes, spectroscopy, and applications. Springer, Cham
Murray PM, Bellany F, Benhamou L et al (2016) The application of design of experiments (DoE) reaction optimisation and solvent selection in the development of new synthetic chemistry. Org Biomol Chem 14:2373–2384
Paredes JI, Villar-Rodil S, Martínez-Alonso A, Tascon JMD (2008) Graphene oxide dispersions in organic solvents. Langmuir 24:10560–10564
Pham VH, Cuong TV, Hur SH et al (2011) Chemical functionalization of graphene sheets by solvothermal reduction of a graphene oxide suspension in N-methyl-2-pyrrolidone. J Mater Chem 21:3371–3377
Marck G, Villiger A, Buchecker R (1994) Aryl couplings with heterogeneous palladium catalysts. Tetrahedron Lett 35:3277–3280
Sakurai H, Tsukuda T, Hirao T (2002) Pd/C as a reusable catalyst for the coupling reaction of halophenols and arylboronic acids in aqueous media. J Org Chem 67:2721–2722
Kitamura Y, Sako S, Udzu T et al (2007) Ligand-free Pd/C-catalyzed Suzuki–Miyaura coupling reaction for the synthesis of heterobiaryl derivatives. Chem Commun. https://doi.org/10.1039/b712207a
Zhou S, Wang M, Yang Z, Zhang X (2019) Polydopamine-modified nanochannels in vertically aligned carbon nanotube arrays for controllable molecule transport. ACS Appl Nano Mater 2:3271–3279
Hemmati S, Mehrazin L, Pirhayati M, Veisi H (2019) Immobilization of palladium nanoparticles on Metformin-functionalized graphene oxide as a heterogeneous and recyclable nanocatalyst for Suzuki coupling reactions and reduction of 4-nitrophenol. Polyhedron 158:414–422
Anuma S, Mishra P, Bhat BR (2019) Copper complex with N-, O-architecture grafted graphene oxide nanosheet as a heterogeneous catalyst for Suzuki cross coupling reaction. J Taiwan Inst Chem Eng 95:643–651
Kim JD, Palani T, Kumar MR et al (2012) Preparation of reusable Ag-decorated graphene oxide catalysts for decarboxylative cycloaddition. J Mater Chem 22:20665–20670
Bowden GD, Pichler BJ, Maurer A (2019) A design of experiments (DoE) approach accelerates the optimization of copper-mediated 18 F-fluorination reactions of arylstannanes. Sci Rep 9:1–10
Lundstedt T, Seifert E, Abramo L et al (1998) Experimental design and optimization. Chemom Intell Lab Syst 42:3–40
Dong L, Wen J, Qin S et al (2012) Iron-catalyzed direct Suzuki–Miyaura reaction: theoretical and experimental studies on the mechanism and the regioselectivity. ACS Catal 2:1829–1837
Kataoka N, Shelby Q, Stambuli JP, Hartwig JF (2002) Air stable, sterically hindered ferrocenyl dialkylphosphines for palladium-catalyzed C-C, C-N, and C-O bond-forming cross-couplings. J Org Chem 67:5553–5566
Beletskaya IP, Cheprakov AV (2000) The Heck reaction as a sharpening stone of palladium catalysis. Chem Rev 100:3009–3066
Yamada YMA, Takeda K, Takahashi H, Ikegami S (2003) Highly active catalyst for the heterogeneous Suzuki–Miyaura reaction: assembled complex of palladium and non-cross-linked amphiphilic polymer. J Org Chem 68:7733–7741
Nájera C, Gil-Moltó J, Karlström S, Falvello LR (2003) Di-2-pyridylmethylamine-based palladium complexes as new catalysts for Heck, Suzuki, and Sonogashira reactions in organic and aqueous solvents. Org Lett 5:1451–1454
Das P, Linert W (2016) Schiff base-derived homogeneous and heterogeneous palladium catalysts for the Suzuki–Miyaura reaction. Coord Chem Rev 311:1–23
Acknowledgements
We are grateful to Bu-Ali-Sina University for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moniriyan, F., Sabounchei, S.J. Comparison of two new graphene-based magnetic and non-magnetic nanocatalysts for Suzuki–Miyaura coupling and optimization of reaction conditions using design of experiment (DoE). Reac Kinet Mech Cat 135, 1803–1818 (2022). https://doi.org/10.1007/s11144-022-02217-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-022-02217-7