Abstract
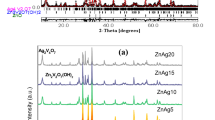
Pristine AgNbO3 and La-doped AgNbO3 perovskite as photocatalysts were successfully synthesized using the solid state method. The synthesized photocatalysts were then characterized by utilising various analytical techniques. The structural characterizations confirmed that the samples are crystalline in nature and without the presence of any secondary phase. The expansion of unit cell parameters from XRD analysis confirmed the inclusion of La into the AgNbO3 lattice structure. FESEM studies revealed irregular morphology of pristine AgNbO3 with the average particle size of 1.42 μm. The particle size decreased as the amount of La increased, with the 10% La-doped AgNbO3 showing the smallest particle size at 0.71 μm. FESEM-EDX confirmed the presence of Ag, Nb, and La atoms. The photocatalytic efficiency of the pristine AgNbO3 and La-doped AgNbO3 were evaluated by performing the photocatalytic degradation of Rhodamine B and methylene blue dyes. The results indicated that La-doping has improved the photocatalytic efficiency of AgNbO3. The increase in efficiency of La-doped AgNbO3 could be attributed to the reduction in particle size and the increased amount of electron–hole pairs being generated during photocatalysis.





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and will be made available on reasonable request.
Code availability
All the codes used are mentioned on page 5.
References
Özgür Ü, Alivov YI, Liu C et al (2005) A comprehensive review of ZnO materials and devices. J Appl Phys 98:1–103. https://doi.org/10.1063/1.1992666
Fortunato E, Barquinha P, Martins R (2012) Oxide semiconductor thin-film transistors: A review of recent advances. Adv Mater 24:2945–2986. https://doi.org/10.1002/adma.201103228
Suryavanshi RD, Mohite SV, Bagade AA, Rajpure KY (2019) Photoelectrocatalytic activity of spray deposited Fe2O3/ZnO photoelectrode for degradation of salicylic acid and methyl orange dye under solar radiation. Mater Sci Eng B Solid-State Mater Adv Technol 248:1–9. https://doi.org/10.1016/j.mseb.2019.114386
Wong LL, Bradt RC (1995) Lime refractories with limestone and synthetic calcium hydroxide additions. J Am Ceram Soc 78:1611–1616. https://doi.org/10.1111/j.1151-2916.1995.tb08859.x
Kusiorowski R (2020) MgO-ZrO2 refractory ceramics based on recycled magnesia-carbon bricks. Constr Build Mater 231:1–12. https://doi.org/10.1016/j.conbuildmat.2019.117084
Diamond JJ, Schneider SJ (1960) Apparent temperatures measured at melting points of some metal oxides in a solar furnace. J Am Ceram Soc 43:1–3. https://doi.org/10.1111/j.1151-2916.1921.tb17340.x
Silakhori M, Jafarian M, Arjomandi M, Nathan GJ (2017) Comparing the thermodynamic potential of alternative liquid metal oxides for the storage of solar thermal energy. Sol Energy 157:251–258. https://doi.org/10.1016/j.solener.2017.08.039
Burda C, Lou Y, Chen X et al (2003) Enhanced nitrogen doping in TiO2 nanoparticles. Nano Lett 3:1049–1051. https://doi.org/10.1021/nl034332o
Lee KM, Lai CW, Ngai KS, Juan JC (2016) Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res 88:428–448. https://doi.org/10.1016/j.watres.2015.09.045
Khan MM, Adil SF, Al-Mayouf A (2015) Metal oxides as photocatalysts. J Saudi Chem Soc 19:462–464. https://doi.org/10.1016/j.jscs.2015.04.003
Watanabe K, Iwase A, Kudo A (2020) Solar water splitting over Rh0.5Cr1.5O3-loaded AgTaO3 of a valence-band-controlled metal oxide photocatalyst. Chem Sci 11:2330–2334. https://doi.org/10.1039/c9sc05909a
Chiarello GL, Dozzi MV, Selli E (2017) TiO2-based materials for photocatalytic hydrogen production. J Energy Chem 26:250–258. https://doi.org/10.1016/j.jechem.2017.02.005
Mokhbi Y, Korichi M, Akchiche Z (2019) Combined photocatalytic and Fenton oxidation for oily wastewater treatment. Appl Water Sci 9:1–9. https://doi.org/10.1007/s13201-019-0916-x
Peng JW, Liu PC, Lee S (2013) Reversible band gap tuning of metal oxide films using hydrogen and oxygen plasmas. Thin Solid Films 531:81–87. https://doi.org/10.1016/j.tsf.2012.12.044
Saboor A, Shah SM, Hussain H (2019) Band gap tuning and applications of ZnO nanorods in hybrid solar cell: Ag-doped verses Nd-doped ZnO nanorods. Mater Sci Semicond Process 93:215–225. https://doi.org/10.1016/j.mssp.2019.01.009
Li JG, Ishigaki T, Sun X (2007) Anatase, brookite, and rutile nanocrystals via redox reactions under mild hydrothermal conditions: Phase-selective synthesis and physicochemical properties. J Phys Chem C 111:4969–4976. https://doi.org/10.1021/jp0673258
Kandiel TA, Feldhoff A, Robben L et al (2010) Tailored titanium dioxide nanomaterials: anatase nanoparticles and brookite nanorods as highly active photocatalysts. Chem Mater 22:2050–2060. https://doi.org/10.1021/cm903472p
Dette C, Pérez-Osorio MA, Kley CS et al (2014) TiO2 anatase with a bandgap in the visible region. Nano Lett 14:6533–6538. https://doi.org/10.1021/nl503131s
Khan MM, Ansari SA, Pradhan D et al (2014) Band gap engineered TiO2 nanoparticles for visible light induced photoelectrochemical and photocatalytic studies. J Mater Chem A 2:637–644. https://doi.org/10.1039/c3ta14052k
Liu R, Yang F, Xie Y, Yu Y (2019) Visible-light responsive boron and nitrogen codoped anatase TiO2 with exposed 0 0 1 facet: Calculation and experiment. Appl Surf Sci 466:568–577. https://doi.org/10.1016/j.apsusc.2018.10.058
Khan MM, Pradhan D, Sohn Y (2017) Nanocomposites for Visible Light-induced Photocatalysis, 1st ed. Springer International Publishing
Khan MM (2021) Chalcogenide-Based Nanomaterials as Photocatalysts, 1st edn. Elsevier
Jiang W, Wang X, Wu Z et al (2015) Silver oxide as superb and stable photocatalyst under visible and near-infrared light irradiation and its photocatalytic mechanism. Ind Eng Chem Res 54:832–841. https://doi.org/10.1021/ie503241k
Wang X, Li S, Yu H et al (2011) Ag2O as a new visible-light photocatalyst: Self-stability and high photocatalytic activity. Chem - A Eur J 17:7777–7780. https://doi.org/10.1002/chem.201101032
Prado AGS, Bolzon LB, Pedroso CP et al (2008) Nb2O5 as efficient and recyclable photocatalyst for indigo carmine degradation. Appl Catal B Environ 82:219–224. https://doi.org/10.1016/j.apcatb.2008.01.024
Souza RP, Freitas TKFS, Domingues FS et al (2016) Photocatalytic activity of TiO2, ZnO and Nb2O5 applied to degradation of textile wastewater. J Photochem Photobiol A Chem 329:9–17. https://doi.org/10.1016/j.jphotochem.2016.06.013
Wang L, Wang L, Li Y et al (2020) Facile fabrication of hierarchical p-Ag2O/n-Nb2O5heterojunction microspheres with enhanced visible-light photocatalytic activity. RSC Adv 10:22432–22439. https://doi.org/10.1039/d0ra04371k
Li G, Yan S, Wang Z et al (2009) Synthesis and visible light photocatalytic property of polyhedron-shaped AgNbO3. J Chem Soc Dalt Trans 3:8519–8524. https://doi.org/10.1039/b906799j
Liu X, Qin C, Huang Y et al (2017) A new silver niobate photocatalyst AgNb13O33: Synthesis, structure and photochemical properties. J Taiwan Inst Chem Eng 78:530–538. https://doi.org/10.1016/j.jtice.2017.06.034
Gao B, Hu D, Xiao C et al (2019) Enhanced visible-light-driven photocatalytic performance of AgNbO3 cubes with a high-energy (001) facet. J Phys Chem Solids 135:1–7. https://doi.org/10.1016/j.jpcs.2019.109083
Tian Y, Jin L, Zhang H et al (2016) High energy density in silver niobate ceramics. J Mater Chem A 4:17279–17287. https://doi.org/10.1039/c6ta06353e
Lu Y, Yu Q, Zhang F et al (2016) Converting Ag nanowire into one-dimensional silver niobate and their enhanced photocatalytic activity. Appl Phys A Mater Sci Process 122:2–6. https://doi.org/10.1007/s00339-016-0367-2
Li G, Kako T, Wang D et al (2009) Enhanced photocatalytic activity of La-doped AgNbO3 under visible light irradiation. J Chem Soc Dalt Trans. https://doi.org/10.1039/b810126d
Ma J, Yan S, Xu C et al (2019) Enhanced energy storage properties of silver niobate ceramics under hydrostatic pressure. Mater Lett 247:40–43. https://doi.org/10.1016/j.matlet.2019.03.035
Chang H, Shang M, Zhang C et al (2012) Hydrothermal syntheses and structural phase transitions of AgNbO3. J Am Ceram Soc 95:3673–3677. https://doi.org/10.1111/j.1551-2916.2012.05392.x
Liu W, Wang H (2011) AgNbO3 ceramics synthesized by aqueous solution-gel method. J Sol-Gel Sci Technol 58:96–101. https://doi.org/10.1007/s10971-010-2361-z
Arney D, Hardy C, Greve B, Maggard PA (2010) Flux synthesis of AgNbO3: Effect of particle surfaces and sizes on photocatalytic activity. J Photochem Photobiol A Chem 214:54–60. https://doi.org/10.1016/j.jphotochem.2010.06.006
Feng X, Lu C, Jia J et al (2020) High temperature tribological behaviors and wear mechanisms of NiAl–NbC–Ag composites formed by in-situ decomposition of AgNbO3. Tribol Int 141:1–9. https://doi.org/10.1016/j.triboint.2019.105898
Levin I, Krayzman V, Woicik JC et al (2009) Structural changes underlying the diffuse dielectric response in AgNbO3. Phys Rev B - Condens Matter Mater Phys 79:1–14. https://doi.org/10.1103/PhysRevB.79.104113
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr Sect A 32:751–767. https://doi.org/10.1107/S0567739476001551
Waterhouse GIN, Bowmaker GA, Metson JB (2001) The thermal decomposition of silver (I, III) oxide: A combined XRD, FT-IR and Raman spectroscopic study. Phys Chem Chem Phys 3:3838–3845. https://doi.org/10.1039/b103226g
Tatsumisago M, Hamada A, Minami T, Tanaka M (1983) Infrared spectra of rapidly quenched glasses in the systems Li2O-RO-Nb2O5 (R=Ba, Ca, Mg). J Am Ceram Soc 66:117–119. https://doi.org/10.1111/j.1151-2916.1983.tb09986.x
Kabir H, Nandyala SH, Rahman MM et al (2018) Influence of calcination on the sol–gel synthesis of lanthanum oxide nanoparticles. Appl Phys A Mater Sci Process 124:1–11. https://doi.org/10.1007/s00339-018-2246-5
Xu R, Tian J, Zhu Q et al (2017) Effects of La-induced phase transition on energy storage and discharge properties of PLZST ferroelectric/antiferroelectric ceramics. Ceram Int 43:13918–13923. https://doi.org/10.1016/j.ceramint.2017.07.120
Xu C, Fu Z, Liu Z et al (2018) La/Mn Codoped AgNbO3 Lead-Free antiferroelectric ceramics with large energy density and power density. ACS Sustain Chem Eng 6:16151–16159. https://doi.org/10.1021/acssuschemeng.8b02821
Athayde DD, Souza DF, Silva AMA et al (2016) Review of perovskite ceramic synthesis and membrane preparation methods. Ceram Int 42:6555–6571. https://doi.org/10.1016/j.ceramint.2016.01.130
Herrera G, Chavira E, Jiménez-Mier J et al (2009) Structural and morphology comparison between m-LaVO4 and LaVO3 compounds prepared by sol-gel acrylamide polymerization and solid state reaction. J Alloys Compd 479:511–519. https://doi.org/10.1016/j.jallcom.2008.12.146
Wheeler GP, Choi KS (2017) Photoelectrochemical properties and stability of nanoporous p-Type LaFeO3 photoelectrodes prepared by electrodeposition. ACS Energy Lett 2:2378–2382. https://doi.org/10.1021/acsenergylett.7b00642
Wang D, Kako T, Ye J (2009) New series of solid-solution semiconductors (AgNbO3)1–x(SrTiO3)x with modulated band structure and enhanced visible-light photocatalytic activity. J Phys Chem C 113:3785–3792. https://doi.org/10.1021/jp807393a
Liu JJ, Fu XL, Chen SF, Zhu YF (2011) Electronic structure and optical properties of Ag3PO4 photocatalyst calculated by hybrid density functional method. Appl Phys Lett 99:1–3. https://doi.org/10.1063/1.3660319
Walsh A, Yan Y, Huda MN et al (2009) Band edge electronic structure of BiVO4: Elucidating the role of the Bi s and V d orbitals. Chem Mater 21:547–551. https://doi.org/10.1021/cm802894z
Zhai Y, Bai X, Pan G et al (2019) Effective blue-violet photoluminescence through lanthanum and fluorine ions co-doping for CsPbCl3 perovskite quantum dots. Nanoscale 11:2484–2491. https://doi.org/10.1039/c8nr09794a
Wan Z, Hu M, Hu B, Yan T (2020) Vacancy induced photocatalytic activity of La doped In(OH)3 for CO2 reduction with water vapor. Catal Sci Technol 10:2893–2904. https://doi.org/10.1039/d0cy00029a
Teixeira GF, Wright TR, Manfroi DC et al (2015) Photoluminescence in NaNbO3 particles and films. Mater Lett 139:443–446. https://doi.org/10.1016/j.matlet.2014.10.088
Lente G (2015) Deterministic Kinetics in Chemistry and Systems Biology. Springer International Publishing, Cham
Wu W, Liang S, Chen Y et al (2013) Mechanism and improvement of the visible light photocatalysis of organic pollutants over microcrystalline AgNbO3 prepared by a sol-gel method. Mater Res Bull 48:1618–1626. https://doi.org/10.1016/j.materresbull.2013.01.011
Ji X, Guo Y, Hua S et al (2020) Interaction-determined sensitization photodegradation of dye complexes by boron nitride under visible light irradiation: Experimental and theoretical studies. New J Chem 44:9238–9247. https://doi.org/10.1039/d0nj01387k
Acknowledgements
The authors would like to thank and acknowledge Universiti Brunei Darussalam, Brunei Darussalam. Abuzar Khan and Mohd Yusuf Khan would like to acknowledge the Interdisciplinary Research Center for Hydrogen and Energy Storage (IRC-HES), Research Institute, King Fahd University of Petroleum & Minerals, KSA for the research support.
Funding
The authors would like to acknowledge the FIC block Grant (UBD/RSCH/1.4/FICBF(b)/2021/035) received from Universiti Brunei Darussalam, Brunei Darussalam.
Author information
Authors and Affiliations
Contributions
CMK methodology, investigation, data curation and writing—original draft. MMK supervision, conceptualization, funding acquisition and writing—review and editing. AK formal analysis and review and editing. MYK formal analysis and review and editing. MHH supervision conceptualization, funding acquisition and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khor, C.M., Khan, M.M., Khan, A. et al. La-substituted AgNbO3 for photocatalytic degradation of Rhodamine B and methylene blue dyes. Reac Kinet Mech Cat 135, 1687–1701 (2022). https://doi.org/10.1007/s11144-022-02199-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-022-02199-6