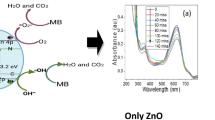
Abstract
Titanium dioxide samples doped with different amounts of dysprosium were synthesized with the sol–gel template method. The structure of the obtained materials was investigated by X-ray fluorescence, scanning electron microscopy, X-ray diffraction (XRD), diffuse reflection spectroscopy, low-temperature adsorption–desorption of nitrogen, and inductively coupled plasma mass spectrometry. According to XRD, it is assumed that Dy3+ ions are statistically distributed in the titanium dioxide phase, predominantly located in interstices or on the surface of TiO2 crystallites. The diffraction patterns show no peaks characteristic of the Dy2O3 phase, the average sizes of crystallites of doped samples decreased compared to the sizes of undoped samples from 16.9 to 7.0–7.6 nm, and the crystal lattice parameters of the obtained materials differ. The introduction of dysprosium into the structure of titanium dioxide reduced the energy of the band gap of the obtained materials from 2.83 to 2.67–2.78 eV, which makes it possible to use them as catalysts for the photooxidation of methyl orange, o- and m-xylenes in water using the visible light. The maximum photocatalytic activity of the oxidation of methyl orange, o- and m-xylenes is characterized by a TiO2 sample containing 9.5% dysprosium (Dy(9.5)/TiO2)—after 2.5 h the degradation of o- and m-xylenes on this catalyst was approximately 80 and 95%, respectively. The degradation of methyl orange on the (Dy(9.5)/TiO2) sample was 73% after 3 h.



Similar content being viewed by others
Data availability
The data is available.
Code availability
Not applicable.
References
Kanan S, Moyet MA, Arthur RB, Patterson HH (2019) Catal Rev 62:1–65. https://doi.org/10.1080/01614940.2019.1613323
Onkani SP, Diagboya PN, Mtunzi FM, Klink MJ, Olu-Owolabi BI, Pakade V (2020) J Environ Manage 260:110145. https://doi.org/10.1016/j.jenvman.2020.110145
Jian Z, Huang S, Cao Y, Zhang Y (2016) Photochem Photobiol 92:363–370. https://doi.org/10.1111/php.12575
Hong X, Tan J, Zhu H, Feng N, Yang Y, Irvine JTS, Wang L, Liu G, Cheng H-M (2019) Chem Eur J 25:1787–1794. https://doi.org/10.1002/chem.201805283
Low J, Cheng B, Yu J (2017) Appl Surf Sci 392:658–686. https://doi.org/10.1016/J.APSUSC.2016.09.093
Tu W, Zhou Y, Liu Q, Yan S, Bao S, Wang X, Xiao M, Zou Z (2012) Adv Funct Mater 23:1743–1749. https://doi.org/10.1002/adfm.201202349
Binas V, Venieri D, Kotzias D, Kiriakidis G (2017) J Materiomics 3:3–16. https://doi.org/10.1016/J.JMAT.2016.11.002
Cha BJ, Saqlain S, Seo HO, Kim YD (2019) Appl Surf Sci 479:31–38. https://doi.org/10.1016/j.apsusc.2019.01.261
Murugan R, Ganesh Ram C (2018) Mater Today: Proc 5:415–421. https://doi.org/10.1016/j.matpr.2017.11.100
Negishi N, Sugasawa M, Miyazaki Y, Hirami Y, Koura S (2019) Water Res 150:40–46. https://doi.org/10.1016/j.watres.2018.11.047
Ferrari-Lima AM, de Souza RP, Mendes SS, Marques RG, Gimenes ML, Fernandez-Machado NRC (2015) Catal Today 241:40–46. https://doi.org/10.1016/j.cattod.2014.03.042
Wang M, Hua J, Yang Y (2018) Spectrochim Acta Part A 199:102–109. https://doi.org/10.1016/j.saa.2018.03.041
Sanchez-Rodriguez D, Medrano MGM, Remita H, Escobar-Barrios V (2018) J Environ Chem Eng 6:1601–1612. https://doi.org/10.1016/j.jece.2018.01.061
Li H, Ji J, Cheng C, Liang K (2018) J Phys Chem Solids 122:25–30. https://doi.org/10.1016/j.jpcs.2018.06.012
Fatima R, Naveed Afridi M, Kumar V, Lee J, Ali I, Kim K-H, Kim J-O (2019) J Clean Prod 231:899–912. https://doi.org/10.1016/j.jclepro.2019.05.292
Unwiset P, Makdee A, Chayakul Chenapattharol K, Kidkhunthod P (2018) J Phys Chem Solids 120:231–240. https://doi.org/10.1016/j.jpcs.2018.05.003
Amorim SM, Suave J, Andrade L, Mendes A, Moreira RFPM (2018) Prog Org Coat 118:48–56. https://doi.org/10.1016/j.porgcoat.2018.01.005
Tbessi I, Benito M, Molins E, Llorca J, Najjar W (2019) Solid State Sci 88:20–28. https://doi.org/10.1016/j.solidstatesciences.2018.12.004
Kumar A, Khan M, Fanf L, Lo IMC (2019) J Hazard Mater 230:108–116. https://doi.org/10.1016/j.jhazmat.2017.07.048
Niaz K, Bahadar H, Maqbool F, Abdollahi M (2015) EXCLI J 14:1167–1186. https://doi.org/10.17179/excli2015-623
Singh RP, Singh PK, Gupta R, Singh RL (2018) Advances in biological treatment of industrial waste water and their recycling for a sustainable future. Springer, Singapore, pp 225–266. https://doi.org/10.1007/978-981-13-1468-1_8
Zhang L, Qin M, Yu W, Zhang Q, Xie H, Sun Z, Shao Q, Guo X, Hao L, Zheng Y, Guo Z (2017) J Electrochem Soc 164:H1086–H1090
Khalid NR, Majid A, Bilal Tahir M, Niaz NA, Khalid S (2017) Ceram Int 43:14552–14571. https://doi.org/10.1016/j.ceramint.2017.08.143
Lv N, Li Y, Huang Z, Li T, Ye S, Dionysios D (2019) Appl Catal B 246:303–311. https://doi.org/10.1016/j.apcatb.2019.01.068
Kim S-G, Dhandole LK, Lim J-M, Chae W-S, Chung H-S, Oh B-T, Jang JS (2018) Appl Catal B 224:791–803. https://doi.org/10.1016/j.apcatb.2017.11.013
Zhou F, Yan C, Wang H, Zhou S, Komarneni S (2019) Mater Lett 228:100–103. https://doi.org/10.1016/j.matlet.2018.05.138
Parnicka P, Mazierski P, Lisowski W, Klimczuk T, Nadolna J, Zaleska-Medynska A (2019) Results Phys 12:412–423. https://doi.org/10.1016/j.rinp.2018.11.073
Zikriya M, Nadaf YF, Vijai Bharathy P, Renuka CG (2019) J Rare Earths 37:24–31. https://doi.org/10.1016/j.jre.2018.05.012
Shafigulin RV, Filippova EO, Shmelev AA, Bulanova AV (2019) Catal Lett 149:916–928. https://doi.org/10.1007/s10562-019-02678-x
Thida SN, Lek S, Rungrote K, Matthana K (2020) Curr Appl Phys 20:249–254. https://doi.org/10.1016/j.cap.2019.11.008
Mousavi M, Soleimani M, Hamzehloo M, Badiei A, Ghasemi JB (2021) Mater Chem Phys 258:123912. https://doi.org/10.1016/j.matchemphys.2020.123912
Mathew S, Ganguly P, Kumaravel V, Harrison J, Hinder JS, Bartlett J, Pillai SC (2020) Mater Today: Proc 33:2458–2464. https://doi.org/10.1016/j.matpr.2020.01.336
Stengl V, Bakardjieva S, Murafa N (2009) Mater Chem Phys 114:217–226. https://doi.org/10.1016/j.matchemphys.2008.09.025
Liu Y, Zhang Q, Xu M, Yuan H, Chen Y, Zhang J, Luo K, Zhang J, You B (2019) Appl Surf Sci 476:632–640. https://doi.org/10.1016/j.apsusc.2019.01.137
Chen Q, Zhang M, Ma Q, Wang Q (2019) J Non-Cryst Solids 507:46–55. https://doi.org/10.1016/j.jnoncrysol.2018.09.025
Lee JY, Choi J-H (2019) Materials 12:1265. https://doi.org/10.3390/ma12081265
Nguyen CH, Fu C-C, Juang R-S (2018) J Clean Prod 202:413–427. https://doi.org/10.1016/j.jclepro.2018.08.110
Zyoud A, Zaatar N, Saadeddin I, Helal MH, Campet G, Hakim M, Park DH, Hilal HS (2011) Solid State Sci 13:1268–1275. https://doi.org/10.1016/j.solidstatesciences.2011.03.020
Lofti H, Heydarinasab A, Mansouri M, Hosseini SH (2022) J Environ Chem Eng 10:107066. https://doi.org/10.1016/j.jece.2021.107066
Azad K, Gajanan P (2017) Chem Sci J 8:1000164. https://doi.org/10.4172/2150-3494.1000164
Funding
This work was supported by the Grant FSSS-2020-0016 within the framework of the state assignment of the Ministry of Education and Science of the Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The authors agree to the publication of the article data.
Research involving humans and/or animals
Not applicable.
Consent to participate
The authors agree to participate.
Consent for publication
The authors agree for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shmelev, A.A., Shafigulin, R.V. & Bulanova, A.V. Dysprosium-doped mesoporous TiO2 as an effective photocatalyst for the oxidation of methyl orange, o- and m-xylenes. Reac Kinet Mech Cat 135, 1047–1058 (2022). https://doi.org/10.1007/s11144-022-02198-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-022-02198-7