Abstract
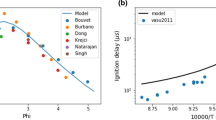
The present study revisited the kinetics of the thermal decomposition and the detonation of H2O2/H2O mixtures in the presence and absence of catalysts aiming to apply in the aerospatial industry. Properties important for the design and optimization of propellant systems, like adiabatic decomposition temperature (Tad), decomposition delay time, Chapman-Jouget velocities, and specific impulses, have been evaluated. Also, aspects related to H2O2 chemistry and its application as a monopropellant are discussed. Three kinetic models were used, and, when appropriate, corrections due to nonideal behavior were included from the Redlich-Kwong equation of state. The modifications due to nonideal behavior can change the calculation of decomposition delay up to 30%. The outcomes show that in the presence or not of catalyst, Tad and specific impulse are maximized at 100% of H2O2. The flow analysis showed that the OH and HO2 radicals are the most important propagators of the thermal decomposition mechanism. For ignition purposes, any proposed catalyst should maximize the concentration of these species during the reaction. The comparison between the simulated burning velocities with the experimental values showed that the modifications proposed in the literature improve the calculation of this property. Regarding the catalysts, SiO2 and Pt are the most prominent since they predict the higher values of adiabatic decomposition temperatures and specific impulse.









Similar content being viewed by others
References
Davis SM, Yilmaz N (2014) Advances in hypergolic propellants: ignition, hydrazine, and hydrogen peroxide research. Adv Aerosp Eng 2014:1–9. https://doi.org/10.1155/2014/729313
Tani H, Terashima H, Daimon Y et al (2018) A numerical study on hypergolic combustion of hydrazine sprays in nitrogen tetroxide streams. Combust Sci Technol 190:515–533. https://doi.org/10.1080/00102202.2017.1402010
Catoire L, Luche J, Dupré G, Paillard C (2001) Critical reactions for the hydrazine vapor detonations. Shock Waves 11:97–103. https://doi.org/10.1007/PL00004070
Konnov AA, De Ruyck J (2001) Kinetic modeling of the decomposition and flames of hydrazine. Combust Flame 124:106–126. https://doi.org/10.1016/S0010-2180(00)00187-5
Sí Ü (2008) Construction of hydrazine and NTO kinetic reaction model for bipropellant thruster simulation. Sp Technol Jpn Jpn Soc Aeronaut Sp Sci 7:1–10. https://doi.org/10.2322/stj.7.1
Maia FF, Pereira LGF, Gouvea LH et al (2014) CoMn-based oxides as bulk catalyst for rocket-grade hydrogen peroxide decomposition. J Propuls Power 30:309–313. https://doi.org/10.2514/1.B34996
Okamoto S, Pereira LGF, Maschio LJ et al (2016) Bulk catalyst for nitrous oxide decomposition in space thrusters. Int J Energ Mater Chem Propuls 15:123–130. https://doi.org/10.1615/IntJEnergeticMaterialsChemProp.2015014682
Schmidt MW, Gordon MS (2013) The decomposition of hydrazine in the gas phase and over an iridium catalyst. Zeitschrift fur Phys Chemie 227:1301–1336. https://doi.org/10.1524/zpch.2013.0404
Al-Shahrani F, Xiao T, Llewellyn SA et al (2007) Desulfurization of diesel via the H2O2 oxidation of aromatic sulfides to sulfones using a tungstate catalyst. Appl Catal B: Environ 73:311–316. https://doi.org/10.1016/J.APCATB.2006.12.016
Chen G-B, Li Y-H, Cheng T-S et al (2011) Effects of hydrogen peroxide on combustion enhancement of premixed methane/air flames. Int J Hydrogen Energy 36:15414–15426. https://doi.org/10.1016/J.IJHYDENE.2011.07.074
Lin S-S, Gurol MD (1998) Catalytic decomposition of hydrogen peroxide on iron oxide: kinetics, mechanism, and implications. Environ Sci Technol 32:1417–1423. https://doi.org/10.1021/es970648k
Zhou J, Schlegel HB (2010) Ab initio molecular dynamics study of the reaction between Th + and H 2 O †. J Phys Chem A 114:8613–8617. https://doi.org/10.1021/jp912098w
Do SH, Batchelor B, Lee HK, Kong SH (2009) Hydrogen peroxide decomposition on manganese oxide (pyrolusite): kinetics, intermediates, and mechanism. Chemosphere 75:8–12. https://doi.org/10.1016/j.chemosphere.2008.11.075
Hong Z, Farooq A, Barbour EA et al (2009) Hydrogen peroxide decomposition rate: a shock tube study using tunable laser absorption of H 2 O near 2.5 μm. J Phys Chem A 113:12919–12925. https://doi.org/10.1021/jp907219f
Hong Z, Cook RD, Davidson DF, Hanson RK (2010) A shock tube study of OH + H 2 O 2 → H 2 O + HO 2 and H 2 O 2 + M → 2OH + M using laser Absorption of H 2 O and OH. J Phys Chem A 114:5718–5727. https://doi.org/10.1021/jp100204z
Hong Z, Lam KY, Sur R et al (2013) On the rate constants of OH + HO2 and HO2+HO 2: a comprehensive study of H2O2 thermal decomposition using multi-species laser absorption. Proc Combust Inst 34:565–571. https://doi.org/10.1016/j.proci.2012.06.108
Zhou H, Shen YF, Wang JY et al (1998) Studies of decomposition of H2O2over manganese oxide octahedral molecular sieve materials. J Catal 176:321–328. https://doi.org/10.1006/jcat.1998.2061
Alfano AJ, Mills JD, Vaghjiani GL (2006) Highly accurate ignition delay apparatus for hypergolic fuel research. Rev Sci Instrum 77:045109. https://doi.org/10.1063/1.2188909
Malik K, Żbikowski M, Bąk D et al (2019) Numerical and experimental investigation of H 2 -air and H 2 –O 2 detonation parameters in a 9 m long tube, introduction of a new detonation model. Int J Hydrogen Energy 44:8743–8750. https://doi.org/10.1016/j.ijhydene.2018.04.161
Haghgoo M, Babaei H, Mostofi TM (2022) 3D numerical investigation of the detonation wave propagation influence on the triangular plate deformation using finite rate chemistry model of LS-DYNA CESE method. Int J Impact Eng 161:104108. https://doi.org/10.1016/J.IJIMPENG.2021.104108
Haghgoo M, Babaei H, Mostofi TM (2021) 2D numerical study on the deflection of thin steel plate subjected to gaseous detonation wave interaction. Int J Hydrogen Energy 46:36348–36368. https://doi.org/10.1016/J.IJHYDENE.2021.08.146
Lin KC, Te CC (2017) A compact skeletal mechanism of propane towards applications from NTC-affected ignition predictions to CFD-modeled diffusion flames: comparisons with experiments. Fuel 203:102–112. https://doi.org/10.1016/j.fuel.2017.04.064
Campbell GA, Rutledge RV (1972) Detonation of hydrogen peroxide vapour. Inst Chem Eng Symp Ser 33:37–43
Engelke R, Sheffield SA, Davis LL (2000) Experimental and predicted detonation parameters for liquid-phase H 2 O 2 /H 2 O mixtures. J Phys Chem A 104:6894–6898. https://doi.org/10.1021/jp000953j
GRI-Mech 3.0. http://combustion.berkeley.edu/gri-mech/version30/text30.html. Accessed 3 Sep 2021
Goodwin DG, Speth RL, Moffat HK, Weber BW (2018) Cantera: an object-oriented software toolkit for chemical kinetics, thermodynamics, and transport processes. https://doi.org/10.5281/zenodo.4527812
Joback KG, Reid RC (1987) Estimation of pure-component properties from group-contributions. Chem Eng Commun 57:233–243. https://doi.org/10.1080/00986448708960487
Liang W, Liu J, Law CK (2017) On explosion limits of H2/CO/O2 mixtures. Combust Flame 179:130–137. https://doi.org/10.1016/J.COMBUSTFLAME.2017.01.024
Wang X, Law CK (2013) An analysis of the explosion limits of hydrogen-oxygen mixtures. J Chem Phys 138:134305. https://doi.org/10.1063/1.4798459
Lee JHS (2008) The detonation phenomenon. Cambridge University Press, Cambridge
Mendiburu Zevallos AA, Ciccarelli G, Carvalho JA Jr (2018) DDT limits of ethanol–air in an obstacles-filled tube. Combust Sci Technol. https://doi.org/10.1080/00102202.2018.1477770
Mével R, Davidenko D, Lafosse F et al (2015) Detonation in hydrogen-nitrous oxide-diluent mixtures: an experimental and numerical study. Combust Flame 162:1638–1649. https://doi.org/10.1016/j.combustflame.2014.11.026
Hitt DL, Zakrzwski CM, Thomas MA (2001) MEMS-based satellite micropropulsion via catalyzed hydrogen peroxide decomposition. Smart Mater Struct 10:1163–1175. https://doi.org/10.1088/0964-1726/10/6/305
An S, Kwon S (2009) Scaling and evaluation of Pt/Al2O3 catalytic reactor for hydrogen peroxide monopropellant thruster. J Propuls Power 25:1041–1045. https://doi.org/10.2514/1.40822
Adami A, Mortazavi M, Nosratollahi M et al (2015) Multidisciplinary design optimization and analysis of hydrazine monopropellant propulsion system. Int J Aerosp Eng 2015:1–9. https://doi.org/10.1155/2015/295636
Explosion Dynamics Laboratory. http://shepherd.caltech.edu/EDL/PublicResources/sdt/. Accessed 2 Oct 2019
Bonifacio S, Sorge AR, Krejci D et al (2014) Novel manufacturing method for hydrogen peroxide catalysts: a performance verification. J Propuls Power 30:299–308. https://doi.org/10.2514/1.B34959
Kang H, Won J, Baek SW, Kwon S (2017) Autoignition and combustion characteristics of sodium borohydride-based non-toxic hypergolic fuel droplet at elevated temperatures. Combust Flame 181:149–156. https://doi.org/10.1016/j.combustflame.2017.03.021
Kang H, Kwon S (2017) Green hypergolic combination: diethylenetriamine-based fuel and hydrogen peroxide. Acta Astronaut 137:25–30. https://doi.org/10.1016/j.actaastro.2017.04.009
Connell TL, Risha GA, Yetter RA, Natan B (2018) Hypergolic ignition of hydrogen peroxide/gel fuel impinging jets. Journal of Propulsion and Power. American Institute of Aeronautics and Astronautics Inc., Reston, pp 182–188
Austin BL, Heister SD, Anderson WE (2005) Characterization of pintle engine performance for nontoxic hypergolic bipropellants. J Propuls Power 21:627–635. https://doi.org/10.2514/1.7988
Papliński A (2019) Explosive potential of energetic mixtures based on hydrogen peroxide. Mater Wysokoenergetyczne/High Energy Mater 11:14–20. https://doi.org/10.22211/matwys/0183
Pourpoint TL, Anderson WE (2007) Hypergolic reaction mechanisms of catalytically promoted fuels with rocket grade hydrogen peroxide. Combust Sci Technol 179:2107–2133. https://doi.org/10.1080/00102200701386149
Bonifacio S, Festa G, Sorge AR (2011) Experimental assessment of hydrogen peroxide decomposition in a monopropellant thruster. Int J Energ Mater Chem Propuls 10:497–522. https://doi.org/10.1615/IntJEnergeticMaterialsChemProp.2012005288
Bonifacio S, Festa G, Russo Sorge A (2012) Catalytic ignition in hydrogen peroxide-based space propulsion systems. In: 48th AIAA/ASME/SAE/ASEE joint propulsion conference & exhibit. American Institute of Aeronautics and Astronautics, Reston, Virigina
Satterfield CN, Kehat E (1961) Burning velocities of the hydrogen peroxide decomposition flame. Combust Flame 5:273–282. https://doi.org/10.1016/0010-2180(61)90106-7
Kee RJ, Coltrin ME, Glarborg P (2005) Chemically reacting flow: theory and practice. 2nd Ed. John Wiley and Sons, 2017
Aul CJ, Crofton MW, Mertens JD, Petersen EL (2011) A diagnostic for measuring H2O2 concentration in a shock tube using tunable laser absorption near 7.8 μm. Proc Combust Inst 33:709–716. https://doi.org/10.1016/J.PROCI.2010.05.063
Kang H, Lee D, Kang S, Kwon S (2017) Effect of H2O2 injection patterns on catalyst bed characteristics. Acta Astronaut 130:75–83. https://doi.org/10.1016/j.actaastro.2016.10.023
Kuznetsov M, Redlinger R, Breitung W et al (2011) Laminar burning velocities of hydrogen-oxygen-steam mixtures at elevated temperatures and pressures. Proc Combust Inst 33:895–903. https://doi.org/10.1016/j.proci.2010.06.050
Das AK, Kumar K, Sung CJ (2011) Laminar flame speeds of moist syngas mixtures. Combust Flame 158:345–353. https://doi.org/10.1016/j.combustflame.2010.09.004
Kumar K, Sung C-J, Hui X (2011) Laminar flame speeds and extinction limits of conventional and alternative jet fuels. Fuel 90:1004–1011. https://doi.org/10.1016/j.fuel.2010.11.022
Galmiche B, Halter F, Foucher F (2012) Effects of high pressure, high temperature and dilution on laminar burning velocities and Markstein lengths of iso-octane/air mixtures. Combust Flame 159:3286–3299. https://doi.org/10.1016/j.combustflame.2012.06.008
Batonneau Y, Brahmi R, Cartoixa B et al (2014) Green propulsion: catalysts for the european FP7 project GRASP. Topics in catalysis. Springer, US, pp 656–667
Cervone A, Torre L, D’Agostino L, et al (2006) Development of hydrogen peroxide monopropellant rockets. In: 42nd AIAA/ASME/SAE/ASEE joint propulsion conference & exhibit. American Institute of Aeronautics and Astronautics, Reston, Virigina, pp 8786–8796
Lee SL, Lee CW (2009) Performance characteristics of silver catalyst bed for hydrogen peroxide. Aerosp Sci Technol 13:12–17. https://doi.org/10.1016/j.ast.2008.02.007
Santos LB, Ribeiro CA, Capela JMV et al (2013) Kinetic parameters for thermal decomposition of hydrazine. J Therm Anal Calorim 113:1209–1216. https://doi.org/10.1007/s10973-013-2968-8
Guseinov SL, Fedorov SG, Kosykh VA, Storozhenko PA (2020) Hydrogen peroxide decomposition catalysts used in rocket engines. Russ J Appl Chem 93:467–487
Hiroki A, LaVerne JA (2005) Decomposition of hydrogen peroxide at water-ceramic oxide interfaces. J Phys Chem B 109:3364–3370. https://doi.org/10.1021/jp046405d
Burcat A (2006) Burcat’s thermodynamic data. http://garfield.chem.elte.hu/Burcat/burcat.html. Accessed 14 Jan 2021
Ventura M, Wernimont E, Heister S, Yuan S (2007) Rocket Grade Hydrogen Peroxide (RGHP) for use in propulsion and power devices - Historical discussion of hazards. In: Collection of Technical Papers - 43rd AIAA/ASME/SAE/ASEE Joint Propulsion Conference 5:4478–4499. https://doi.org/10.2514/6.2007-5468
Acknowledgements
The authors thanks to FAPERJ for the financial support of this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Ferreira Miranda, A.F., Bauerfeldt, G.F. & Baptista, L. Numerical simulation of the gas-phase thermal decomposition and the detonation of H2O2/H2O mixtures. Reac Kinet Mech Cat 135, 619–637 (2022). https://doi.org/10.1007/s11144-022-02161-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-022-02161-6