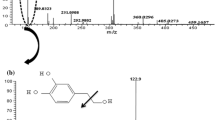
Abstract
The present research work undertakes the investigation of an effective and inexpensive process to produce hydroxytyrosol, a naturally occurring orthodiphenolic antioxidant molecule found in olive oil from its monophenolic precursor tyrosol. This conversion was achieved from the wet hydrogen peroxide catalytic oxidation with montmorillonite KSF as economical and environment-friendly solid acid at room temperature. The impact of the primary functional parameters comprising tyrosol, H2O2, catalyst concentration and the interaction between them were performed using an experimental design Box-Behnken methodology. The percentage of 80% of tyrosol was converted, under the optimized operating conditions with higher hydroxytyrosol selectivity up to 90%. A kinetics of a pseudo first order pertaining to the tyrosol concentration was noticed. Montmorillonite KSF shows excellent stability and can be reused in three runs without significant activity decrease. The proposed procedure, investigated for the first time, is operationally simple and could find applications for industrial purposes.
Graphical abstract









Similar content being viewed by others
References
Cañuelo A, Gilbert-López B, Pacheco-Liñán P et al (2012) Tyrosol, a main phenol present in extra virgin olive oil, increases lifespan and stress resistance in Caenorhabditis elegans. Mech Ageing Dev 133:563–574
Carrasco-Pancorbo A, Cerretani L, Bendini A et al (2005) Evaluation of the antioxidant capacity of individual phenolic compounds in virgin olive oil. J Agric Food Chem 53:8918–8925
Mateos R, Martínez López S, Baeza Arévalo G et al (2016) Hydroxytyrosol in functional hydroxytyrosol-enriched biscuits is highly bioavailable and decreases oxidised low density lipoprotein levels in humans. Food Chem 205:248–256
Kitsati N, Mantzaris MD, Galaris D (2016) Hydroxytyrosol inhibits hydrogen peroxide-induced apoptotic signaling via labile iron chelation. Redox Biol 10:233–242
Anter J, Tasset I, Demyda-Peyrás S et al (2014) Evaluation of potential antigenotoxic, cytotoxic and proapoptotic effects of the olive oil by-product “alperujo”, hydroxytyrosol, tyrosol and verbascoside. Mutat Res Genet Toxicol Environ Mutagen 772:25–33
Hassan S, Eid D (2016) Effect of tyrosol on Staphylococcus aureus antimicrobial susceptibility, biofilm formation and virulence factors. Afr J Microbiol Res 10:687–693
Karković Marković A, Torić J, Barbarić M et al (2019) Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules 24:2001
Atochin Dmitriy N, Chernysheva Galina A, Vera S et al (2016) Neuroprotective effects of p-tyrosol after the global cerebral ischemia in rats. Phytomedicine 23:784–792
Belmonte-Reche E, Martínez-García M, Peñalver P et al (2016) Tyrosol and hydroxytyrosol derivatives as antitrypanosomal and antileishmanial agents. Eur J Med Chem 119:132–140
Tejada S, Pinya S, Del Mar BM et al (2017) Cardioprotective effects of the polyphenol hydroxytyrosol from olive oil. Curr Drug Targets 18:477–1486
Robles-Almazan M, Pulido-Moran M, Moreno-Fernandez J et al (2018) Hydroxytyrosol: bioavailability, toxicity, and clinical applications. Food Res Int 105:654–667
Chaozhi L, Pu J, Yajun B et al (2019) Efficient synthesis of hydroxytyrosol from l-3,4-dihydroxyphenylalanine using engineered escherichia coli whole cells. J Agric Food Chem 67:6867–6873
Santos MM, Piccirillo C, Castro PML et al (2012) Bioconversion of oleuropein to hydroxytyrosol by lactic acid bacteria. World J Microbiol Biotechnol 28:2435–2440
Orenes-Piñero E, García-Carmona F, Sánchez-Ferrer Á (2013) A new process for obtaining hydroxytyrosol using transformed Escherichia coli whole cells with phenol hydroxylase gene from Geobacillus thermoglucosidasius. Food Chem 139:377–383
Hye Jeong C, Eun Ji K, So Yeon K et al (2018) Microbial synthesis of hydroxytyrosol and hydroxysalidroside. J Appl Biol Chem 61:295–301
Rodríguez-Morató J, Robledo P, Farré M et al (2015) Evaluation of the Pathways Involved in the in vivo Biotransformation of Tyrosol into Hydroxytyrosol. The FASEB J 29.
Azabou S, Najjar W, Ghorbel A et al (2007) A mild photochemical synthesis of the antioxidant hydroxytyrosol via conversion of tyrosol. J Agric Food Chem 55:4877–4882
Ben Abdallah F, Hmani E, Bouaziz M et al (2017) Recovery of hydroxytyrosol a high added value compound through tyrosol conversion by electro-Fenton process. Sep Purif Technol 188(29):260–265
Bouguerra Neji S, Trabelsi M, Frikha MH (2011) Esterification of fatty acid over tunisian acid activated clay: kinetic study. J Oleo Sci 60:293–299
Bouguerra Neji S, Azabou S, Contreras S et al (2016) Novel mild synthesis of high-added-value p-hydroxyphenyl acetic acid and 3,4-dihydroxyphenyl acetic acid using the acidic clay/hydrogen peroxide catalytic system. C R Chim 19:286–292
Kite GC, Stoneham CA, Veitch NC (2007) Flavonol tetraglycosides and other constituents from leaves of Styphnolobium japonicum (Leguminosae) and related taxa. Phytochemistry 681407–1416.
Salgado P, Melin V, Contreras D et al (2013) Fenton reaction driven by iron ligands. J Chil Chem Soc 58:1842–1847
Hafiidz Mohammad Fauzi A, Aishah Saidina Amin N (2013) Optimization of oleic acid esterification catalyzed by ionic liquid for green biodiesel synthesis. Energ Convers Manage 76:818–827
Ben Hmida R, Frikha N, Bouguerra S (2020) Synthesis of high added value compounds through catalytic oxidation of 2-phenylethanol: A Kinetic study. Int J Chem Kinet 52:124–133
Sirés I, Brillas E, Oturan MA et al (2014) Electrochemical advanced oxidation processes: today and tomorrow. Environ Sci Pollut Res 21:8336–8367
Wei X, Wu H, Sun F (2017) Magnetite/Fe-Al-montmorillonite as a Fenton catalyst with efficient degradation of phenol. J Colloid Interface Sci 504:611–619
Gümüş D, Akbal F (2016) Comparison of Fenton and electro-Fenton processes for oxidation of phenol. Process Saf Environ Prot 103:252–258
Ma L, Zhou M, Ren G et al (2016) A highly energy-efficient flow-through electro-Fenton process for organic pollutants degradation. Electrochim Acta 200:222–230
Frikha N, Bouguerra S, Ammar S et al (2019) Kinetics and mechanism of the oxidation of vanillic acid using smectite clay. React Kinet Mech Catal 128:903–916
Frikha N, Bouguerra S, Kit G et al (2019) Smectite clay KSF as effective catalyst for oxidation of m-tyrosol with H2O2 to hydroxytyrosol. React Kinet Mech Catal 127:505–521
Lee HV, Yunus R, Juan JC et al (2011) Process optimization design for jatropha-based biodiesel production using response surface methodology. Fuel ProcessTechnol 92:2420–2428
Oturan MA, Oturan N, Lahite C et al (2001) Production of hydroxyl radicals by electrochemically assisted Fenton’s reagent. Application to the mineralization of an organic micropollutant, pentachlorophenol. J Electroanal Chem 507:96–102
Brillas E, Banos M, Garrido J (2003) Mineralization of herbicide 3,6-dichloro-2-methoxybenzoic acid in aqueous media by anodic oxidation, electro-Fenton and photoelectro-Fenton. Electrochim Acta 48:1697–1705
Rekik R, Hamza M, Jaziri M et al (2020) Electrochemical oxidation of vanillic acid by electro-Fenton process: toward a novel route of protocatechuic acid electrosynthesis. Arab J Chem 13:357–365
Schweigert N, Zehnder AJ, Eggen RI. Chemical properties of catechols and their molecular modes of toxic action in cells, from microorganisms to mammals. Environ. Microbiol 3:81–91.
Binbuga N, Chambers K, Henry WP et al (2005) Metal chelation studies relevant to wood preservation.1. Complexation of propyl gallate with Fe2+. Holzforschung 59:205–209
Mijangos F, Varona F, Villota N (2006) Changes in solution color during phenol oxidation by Fenton reagent. Environ Sci Technol 40:5538–5543
Tofan-Lazar J, Situm A, Al-Abadleh HA (2013) DRIFTS studies on the role of surface water in stabilizing catechol-iron (III) complexes at the gas/solid interface. Am J Phys Chem 117:10368–11038
John M, Baloyi J, Nthob T (2018) Synthesis and characterization of an efficient and stable Al/Fe pillared clay catalyst for the catalytic wet air oxidation of phenol. RSC Adv 8:30115–30124
Acknowledgements
This research work was supported by the Tunisian Ministry of Higher Education and Scientific Research. The authors would like to acknowledge the significant contribution of all the experts who participated in this study: M. Fatma Masmoudi for the experimental design, Dr. Nourezed Frikha for HPLC and LC-MS analysis. They also extend their thanks to Olfa Gharbi and Jihed Ben Amor for their contribution.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bouguerra Neji, S., Bouaziz, M. Optimization of a novel method for the conversion of tyrosol to hydroxytyrosol via catalytic process using statistical experimental design: kinetic study. Reac Kinet Mech Cat 135, 201–217 (2022). https://doi.org/10.1007/s11144-021-02126-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-02126-1