Abstract
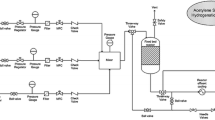
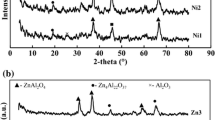
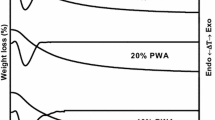
Selective hydrogenation of acetylene was studied using palladium catalysts supported on different transition alumina spheres. The alumina supports were prepared by calcining pseudo boehmite alumina sphere at different calcination temperatures; the ρ-Al2O3 used was flash calcined alumina. The alumina supports were characterized using different techniques like XRD, surface area, pore volume and acidity. The Pd/Al2O3 catalyst were prepared, palladium penetration depth was measured using microscopic imaging and the catalyst reducibility were studied by Temperature Programmed Reduction (TPR). The microscopic imaging using TEM were done to study the palladium particle size and nature of particle clusters on the support surface. The catalyst performance evaluation was done in a fixed bed reactor with 1% acetylene in nitrogen feed and studied the impact of support properties on the catalytic activity. The catalyst prepared on γ-Al2O3 support showed the highest conversion and lowest selectivity, whereas the catalyst on α-Al2O3 support has the lower conversion and highest selectivity. Thermogravimetric analysis coupled with mass spectroscopy analysis were done for the spent catalyst to understand the possibilities of heavy component deposition of the catalyst. The catalyst prepared on α-Al2O3 support had the lowest hydrocarbon deposit on the surface whereas the γ and δ alumina showed the highest hydrocarbon deposits on the catalyst surface.









Similar content being viewed by others
References
Tiedtke DB, Bergmeister JJ, Cheung TP, Rhoades RA (2001) Progress in the development of E-Series catalyst technologies for the selective hydrogenation of acetylene in various hydrocarbon streams. Thai Olefins Ethylene Technology Forum
McCue AJ, Anderson JA (2015) Recent advances in selective acetylene hydrogenation using palladium containing catalysts. Front Chem Sci Eng 9:142–153. https://doi.org/10.1007/s11705-015-1516-4
Takht Ravanchi M, Fadaeerayeni S, Rahimi Fard M (2014) An egg-shell Pd-Ag/α-Al2O3 catalyst for tail-end acetylene selective hydrogenation. IJChE 11(1):42–54
Sivaraj CH, Contescue CR, Schwarz JA (1991) Effect of calcination temperature of alumina on the adsorption/impregnation of Pd(II) compounds. J Catal 132(2):422–431. https://doi.org/10.1016/0021-9517(91)90159-2
Hill MR, Bastow TJ, Celotto S, Hill AJ (2007) Integrated study of the calcination cycle from gibbsite to corundum. Chem Mater 19(11):2877–2883. https://doi.org/10.1021/cm070078f
Boumaza A, Favaro L, Lédion J et al (2009) Transition alumina phases induced by heat treatment of boehmite: An X-ray diffraction and infrared spectroscopy study. J Solid State Chem 182(5):1171–1176. https://doi.org/10.1016/j.jssc.2009.02.006
Takht Ravanchi M, Rahimi Fard M, Fadaeerayeeni S, Yaripour F (2015) Effect of calcination conditions on crystalline structure and pore size distribution for a mesoporous alumina. Chem Eng Commun 202(4):493–499. https://doi.org/10.1080/00986445.2013.850577
Chang PL, Yen FS, Cheng KC, Wen HL (2001) Examinations on critical and primary crystallite sizes during θ- to α-phase transformations of ultrafine alumina powders. Nano Lett 1(5):253–261. https://doi.org/10.1021/nl015501c
Iaponeide M, Macedo F, Aparecido Bertran C, Osawa CC (2007) Kinetic of the γ-to α-alumina transformation by quantitative X-ray diffraction. J Mater Sci 42:2830–2836. https://doi.org/10.1007/s10853-006-1364-1
Perander LM, Zujovic ZD, Groutso T, Hyland MM, Smith ME, O’Dell LA, Metson JB (2007) Characterization of metallurgical-grade aluminas and their precursors by 27Al NMR and XRD. Can J Chem 85(10):889–897. https://doi.org/10.1139/v07-106
Application note 134 https://www.micromeritics.com/Repository/Files/apnote134.pdf.
Gopinath R, Babu NS, Kumar JV, Lingaiah N, Prasad PS (2008) Influence of Pd precursor and method of preparation on hydrodechlorination activity of alumina supported palladium catalysts. Catal Lett 120:312–319. https://doi.org/10.1007/s10562-007-9287-2
Zhu Q, Gao J, Chen J, Wen L (2010) Selective hydrogenation of acetylene over egg-shell palladium nanocatalyst. J Nanosci Nanotechnol 10(9):5641–5647. https://doi.org/10.1166/jnn.2010.2472
Storozhenko PA, Aleshin AI, Douganyuk VF (2010) Selecting heat treatment conditions of alumina supports to improve the quality of selective hydrogenation palladium catalysts. Catal Ind 2:282–286. https://doi.org/10.1134/S207005041003013X
Khan NA, Shaikhutdinov S, Freund HJ (2006) Acetylene and ethylene hydrogenation on alumina supported Pd-Ag model catalysts. Catal Lett 108:159–164. https://doi.org/10.1007/s10562-006-0041-y
Ludwig W, Savara AA, Dostert K-H, Schauermann S (2011) Olefin hydrogenation on Pd model supported catalysts: new mechanistic insights. J Catal 284(2):148–156. https://doi.org/10.1016/j.jcat.2011.10.010
Paryjczak T, Rynkowski J (1984) Temperature-programmed reduction and temperature-programmed oxidation of nickel and copper-nickel catalysts with addition of palladium supported on alumina. React Kinet Mech Cat 24:187–191
Application note 120 “Temperature-Programmed Reduction Using the AutoChem” https://www.micromeritics.com/Repository/Files/appnote120.pdf
Sorbier L, Gay A-S, Fécant A, Moreaud M, Brodusch N (2013) Measurement of palladium crust thickness on catalysts by optical microscopy and image analysis. Microsc Microanal 19(2):293–299. https://doi.org/10.1017/S1431927612014316
Dehghani O, Rahimpour MR, Shariati A (2019) An experimental approach on industrial Pd-Ag supported α-Al2O3 catalyst used in acetylene hydrogenation process: mechanism, kinetic and catalyst decay. MDPI Process 27(3):136. https://doi.org/10.3390/pr7030136
Takht Ravanchi M, Fadaeerayeni S, Rahimi Fard M (2017) Acetylene selective hydrogenation: a technical review on catalytic aspects. Rev Chem Eng 34(2):215–237. https://doi.org/10.1515/REVCE-2016-0036
Komhom S, Mekasuwandumrong O, Praserthdam P, Panpranot J (2008) Improvement of Pd/Al2O3 catalyst performance in selective acetylene hydrogenation using mixed phases Al2O3 support. Catal Commun 10(1):86–91. https://doi.org/10.1016/j.catcom.2008.07.039
Taromi AA, Kaliaguine S (2017) Synthesis of ordered mesoporous γ-alumina: effects of calcination conditions and polymeric template concentration. Microporous Mesoporous Mater 248:179–191. https://doi.org/10.1016/j.micromeso.2017.04.040
Macêdo MIF, Bertran CA, Osawa CC (2007) Kinetics of the γ → α-alumina phase transformation by quantitative X-ray diffraction. J Mater Sci 42:2830–2836. https://doi.org/10.1007/s10853-006-1364-1
Ravanchi MT, Fadaeerayeni S, Fard MR (2016) The effect of calcination temperature on physicochemical properties of alumina as a support for acetylene selective hydrogenation catalyst. Res Chem Intermed 4(2):4797–4811. https://doi.org/10.1007/s11164-015-2320-y
Sung DM, Kim YH, Park ED, Yie JE (2010) Correlation between acidity and catalytic activity for the methanol dehydration over various aluminum oxides. Res Chem Intermed 36:653–660. https://doi.org/10.1007/s11164-010-0201-y
Standard Practice for Calculation of Pore Size Distributions of Catalysts and Catalyst Carriers from Nitrogen Desorption Isotherms, ASTM D4641-1. https://www.quantachrome.com/standards.html
Vaidya SD, Thakkar NV (2001) Effect of temperature, pH and ageing time on hydration of rho alumina by studying phase composition and surface properties of transition alumina obtained after thermal dehydration. Mater Lett 51(4):295–300. https://doi.org/10.1016/S0167-577X(01)00307-X
Bhogeswararao S, Srinivas D (2015) Catalytic conversion of furfural to industrial chemicals over supported Pt and Pd catalysts. J Catal 327:65–77. https://doi.org/10.1016/j.jcat.2015.04.018
Wongwaranom N, Mekasuwandumrong O, Praserthdam P, Panpranot P (2008) Performance of Pd catalysts supported on nanocrystalline α-Al2O3 and Ni-modified α-Al2O3 in selective hydrogenation of acetylene. Catal Today 131(1–4):553–558. https://doi.org/10.1016/j.cattod.2007.10.036
Duca D, Varga Z, La Manna G, Vidoczy T (2000) Hydrogenation of acetylene-ethylene mixtures on Pd catalysts: study of the surface mechanism by computational approaches, metal dispersion and activity of the catalyst. Theor Chem Acc 104:302–311. https://doi.org/10.1007/s002140000123
Komeilia S, Ravanchib MT, Taeb A (2015) The influence of alumina phases on the performance of the Pd–Ag/Al2O3catalyst in tail-end selective hydrogenation of acetylene. Appl Catal A 502:287–296. https://doi.org/10.1016/j.apcata.2015.06.013
Bos ANR, Westerterp KR (1993) Mechanism and kinetics of the selective hydrogenation of ethyne and ethane. Chem Eng Process 32(1):1–7. https://doi.org/10.1016/0255-2701(93)87001-B
Gislason J, Xia W, Sellers H (2002) Selective hydrogenation of acetylene in an ethylene rich flow: results of kinetic simulation. J Phys Chem 106(5):767–774. https://doi.org/10.1021/jp011238s
Battiston GC, Dalloro L, Tauszik GR (1982) Performance and aging of catalysts for the selective hydrogenation of acetylene: a micropilot-plant study. Appl Catal 2(1–2):1–17. https://doi.org/10.1016/0166-9834(82)80170-X
Ball MR, Rivera-Dones KR, Gilcher EB, Ausman SF, Hullfish CW, Lebrón EA, Dumesic JA (2020) AgPd and CuPd catalysts for selective hydrogenation of acetylene. ACS Catal 10(15):8567–8581. https://doi.org/10.1021/acscatal.0c01536
HeupMoon S-J (2011) Performance of Cu-promoted Pd catalysts prepared by adding Cu using a surface redox method in acetylene hydrogenation. Appl Catal A 401(1–2):12–19. https://doi.org/10.1016/j.apcata.2011.04.048
Zou S et al (2021) Grafting nanometer metal/oxide interface towards enhanced low-temperature acetylene semi-hydrogenation. Nat Commun 12:5570. https://doi.org/10.1038/s41467-021-25984-8
Feng J-T, Ma X-Y, Evans DG, Li D-Q (2011) Enhancement of metal dispersion and selective acetylene hydrogenation catalytic properties of a supported Pd catalyst. Ind Eng Chem Res 50(4):1947–1954. https://doi.org/10.1021/ie101508z
Ibhadon AO, Kansal SK (2018) The reduction of alkynes over Pd-based catalyst materials: a pathway to chemical synthesis. J Chem Eng Process Technol. https://doi.org/10.4172/2157-7048.1000376
Pradier CM, Mazina M, Berthier Y, Oudar J (1994) Hydrogenation of acetylene on palladium. J Mol Catal 89(1–2):20211–20220. https://doi.org/10.1016/0304-5102(93)E0323-9
Ravanchi MT, Sahebdelfar S, Komeili S (2018) Acetylene selective hydrogenation: a technical review on catalytic aspects. Rev Chem Eng 34(2):215–237. https://doi.org/10.1515/revce-2016-0036
Ravanchi MT, Sahebdelfar S, Fard MR, Fadaeerayeni S, Bigdeli P (2016) Pd-Ag/α-Al2O3 catalyst deactivation in acetylene selective hydrogenation. Chem Eng Process 39(2):301–310. https://doi.org/10.1002/ceat.201400526
Wang Z, Du W, Qian F, Tian L, Jiang D (2015) Improve acetylene hydrogenation selectivity using dynamic deactivation estimation. Hydrocarbon processing. Gulf Publishing Company, Houston
Kuhn M, Lucas M, Claus P (2015) Precise recognition of catalyst deactivation during acetylene hydrogenation studied with the advanced TEMKIN reactor. Catal Commun 72(5):170–173. https://doi.org/10.1016/j.catcom.2015.10.001
Samavatia M, Ebrahima HA, Dorjb Y (2018) Effect of the operating parameters on the simulation of acetylene hydrogenation reactor with catalyst deactivation. Appl Catal A 567:45–55. https://doi.org/10.1016/j.apcata.2018.06.038
Acknowledgements
The author (KR) acknowledges the support received from the Management and R&D colleagues of Sud-Chemie India (P) Ltd for all the support received in completing this study. The author acknowledges DST-SAIF Cochin for the microscopic data analysis.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
KR—main contributor for literature search, design, execution of the experiments and data interpretations involved in this study. GM—supervisor and research guide. VRR—contributed to the design and safety aspects of the test unit and the process. SR—contributed for the review of thermogravimetric studies and spent catalyst analysis.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interests.
Consent to participate
All authors participated in the creation of this manuscript.
Consent for publication
All authors agree to publish this manuscript in the journal.
Availability of data and materials
The authors confirm that the data and the findings mentioned in this study are available within the article and raw data and the derived data supporting the findings are available from the corresponding author on request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ravindran, K., Madhu, G., Renjith, V.R. et al. Influence of different alumina phases on the catalytic properties of palladium-alumina catalysts for selective hydrogenation of acetylene to ethylene. Reac Kinet Mech Cat 134, 867–882 (2021). https://doi.org/10.1007/s11144-021-02112-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-02112-7