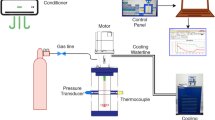
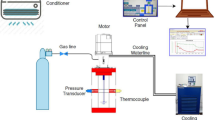
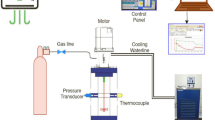
Abstract
This study examines the impact of three amino acids such as proline, methionine and tryptophan on methane (95%)–propane (5%) hydrate formation with the use of different impellers. The concentration of amino acids was 1 wt% at 24.5 bar and 2 °C. Based on experimental outcomes proline behaves as inhibitor and methionine and tryptophan perform as promoters. RT experiments both formed more quickly gas hydrates and indicated higher values in rate of hydrate formation compared to PBTU and PBTD experiments showing that in radial flow bubbles are subjected to higher shear stresses, their size are reduced, so that the contact surface is increased resulting in an improved mass transfer coefficient.





Similar content being viewed by others
Abbreviations
- LNG:
-
Liquefied natural gas
- KHI:
-
Kinetic hydrate inhibitors
- CNG:
-
Compressed natural gas
- LDHI:
-
Low dosage hydrate inhibitors
- THI:
-
Thermodynamic hydrate inhibitor
- AA:
-
Anti agglomerates
- MEG:
-
Monoethylene glycol
- SDS:
-
Sodium dodecyl sulfite
- TBAB:
-
Tetra-n-butyl ammonium-bromide
- PVP:
-
Polyvinylpyrrolidone
- CO2 :
-
Carbon dioxide
- LABS:
-
Linear alkyl benzene sulfonate
- DBSA:
-
Dodecyl benzene sulfonic acid
- SDSN:
-
Sodium dodecyl sulfonate
- LDS:
-
Lithium dodecyl sulfate
- SO:
-
Sodium oleate
- SHS:
-
Sodium hexadecyl sulfate
- SDBS:
-
Sodium dodecyl benzene sulfonate
- STS:
-
Sodium tetracyl sulfate
- DAH:
-
Dodecylamine hydrochloride
- HTABr:
-
Hexadecyl-trimethyl-ammoium bromide
- CTAB:
-
Cetyl trimethyl ammonium bromide
- ENP:
-
Ethoxylated nonylphenol
- DN2CL:
-
N-Dodecylpropane-1,3-diamine hydrochloride
- PBTU:
-
Pitched blade turbine upward
- PBTD:
-
Pitched blade turbine downward
- RT:
-
Rushton turbine
- dn/dt:
-
Gas consumption rate mol/s
- t:
-
Time, s
- NR30 :
-
Hydrate productivity mol/s × l
- R30 :
-
Hydrate formation for first 30 min, mol/s
- Vwater :
-
Volume of experiment
References
Longinos SN, Longinou DD, Achinas S (2020) Natural gas hydrates: possible environmental issues. Contemporary environmental issues and challenges in era of climate change. Springer, Singapore
Longinos SN, Bulbul S, Parlaktuna M (2019) Potential effects of methane hydrates to the environment, PESXM-12th Pan-Hellenic scientific conference in chemical engineering. Greece, Athens
Longinos S (2019) Potential environmental challenges for gas hydrates. LAP Lambert Academic Publishing, Beau Basin
Gudmundsson J, Borrehaug A (1996) Frozen hydrate for transport of natural gas. In: NGH 96: 2nd international conference on natural Gaz hydrates (Toulouse, June 2–6, 1996), pp 415–422
Shirota H, Aya I, Namie J (2002) Measurements of methane hydrate dissociation for application to natural gas storage and transportation. In: Proceedings of the fourth international conference on natural gas hydrates, Yokohama, pp 972–977
Akhmetzhan A, Abeu N, Longinos SN, Tashenov A, Myrzakhmetova N, Amangeldi N, Kuanyshova Z, Ospanova Z, Toktarbay Z (2021) Synthesis and heavy metal sorption studies of N,N-dimethylacrylamide based hydrogels. Polymers 13(18):3084. https://doi.org/10.3390/polym13183084
Dauletov Y, Nuraje N, Abdiyev K, Toktarbay Z, Zhursumbaeva M (2019) Copolymers of diallyldimethylammonium chloride and vinyl ether of monoethanolamine: synthesis, flocculating, and antimicrobial properties. J Surfactants Deterg 22(5):1129–1137
Bai Y, Bai Q (2005) Hydrates, Subsea Pipelines and Rivers, Ocean Engineering Series. Elsevier Ltd, The Boulevard, Langford Lane, Kidlington, Oxford, UK, 357–38
Merey S, Longinos SN (2019) The gas hydrate potential of the eastern Mediterranean sea. Bull Min Res Exp 160:117–134. https://doi.org/10.19111/bulletinofmre.502275
Sloan ED (2001) Hydrate engineering, vol 21. SPE, Richardson
Merey S, Longinos SN (2018) The role of natural gas hydrates during natural gas transportation. OHU J Eng Sci 7(2):937–953. https://doi.org/10.28948/ngumuh.445410
Sloan ED, Koh CA, Sum A (2011) Natural gas hydrate in flow assurance. Gulf Professional Publishing, New York
Wang W, Li Y, Liu H, Zhao P (2015) Study of agglomeration characteristics of hydrate particles in oil/gas pipelines. Adv Mech Eng 7(1):457050
Merey S, Longinos SN (2018) Investigation of gas seepages in Thessaloniki mud volcano in the Mediterranean Sea. J Petrol Sci Eng 168:81–97. https://doi.org/10.1016/j.petrol.2018.05.014
Merey S, Longinos SN (2018) Does the Mediterranean sea have potential for producing gas hydrates? J Nat Gas Sci Eng 55:113–134. https://doi.org/10.1016/j.jngse.2018.04.029
Abdiyev KZ, Toktarbay Z, Zhenissova AZ, Zhursumbaeva MB, Kainazarova RN (2015) Copolymerization of N, N-dimethyl-N, N-diallylammonium chloride with N,N-dimethylacrylamide. Polym Sci Ser B 57(3):217–223
Anderson FE, Prausnitz JM (1986) Inhibition of gas hydrates by methanol. AIChE J 32(8):1321–1333
Akhfash M, Arjmandi M, Aman ZM, Boxall JA, May EF (2017) Gas hydrate thermodynamic inhibition with MDEA for reduced MEG circulation. J Chem Eng Data 62(9):2578–2583
Ayatzhan A, Tashenov A, Nurgeldi A, Zhanar O, Zhexenbek T, Kaldibek A, Nuraje N (2021) P (DADMAAC-co-DMAA): synthesis, thermal stability, and kinetics. Polym Adv Technol 32(7):2669–2675
Mohammadi AH, Afzal W, Richon D (2008) Experimental data and predictions of dissociation conditions for ethane and propane simple hydrates in the presence of methanol, ethylene glycol, and triethylene glycol aqueous solutions. J Chem Eng Data 53(3):683–686
Dauletov Y, Abdiyev K, Toktarbay Z, Nuraje N, Zhursumbaeva M, Kenzhaliyev B (2018) Radical polymerization and kinetics of N, N-diallyl-N, N-dimethylammonium chloride and vinyl ether of monoethanolamine. Fibers Polym 19(10):2023–2029
Jensen L, Thomsen K, von Solms N (2011) Inhibition of structure I and II gas hydrates using synthetic and biological kinetic inhibitors. Energy Fuels 25(1):17–23
Kinnari K, Hundseid J, Li X, Askvik KM (2015) Hydrate management in practice. J Chem Eng Data 60(2):437–446
Kelland MA (2006) History of the development of low dosage hydrate inhibitors. Energy Fuels 20(3):825–847
Ke W, Kelland MA (2016) Kinetic hydrate inhibitor studies for gas hydrate systems: a review of experimental equipment and test methods. Energy Fuels 30(12):10015–10028
Kvamme BB, Huseby G, Forrisdahl OK (1997) Molecular dynamics simulations of PVP kinetic inhibitor in liquid water and hydrate/liquid water systems. Mol Phys 90(6):979–992
Kvamme B, Kuznetsova T, Aasoldsen K (2005) Molecular dynamics simulations for selection of kinetic hydrate inhibitors. J Mol Graph Model 23(6):524–536
Ajiro H, Takemoto Y, Akashi M, Chua PC, Kelland MA (2010) Study of the kinetic hydrate inhibitor performance of a series of poly (N-alkyl-N-vinylacetamide)s. Energy Fuels 24(12):6400–6410
Chua PC, Sæbø M, Lunde A, Kelland MA (2011) Dual kinetic hydrate inhibition and scale inhibition by polyaspartamides. Energy Fuels 25(11):5165–5172
Villano LD, Kommedal R, Fijten MW, Schubert US, Hoogenboom R, Kelland MA (2009) A study of the kinetic hydrate inhibitor performance and seawater biodegradability of a series of poly (2-alkyl-2-oxazoline) s. Energy Fuels 23(7):3665–3673
Veluswamy HP, Kumar A, Seo Y, Lee JD, Linga P (2018) A review of solidified natural gas (SNG) technology for gas storage via clathrate hydrates. Appl Energy 216:262–285
Kalogerakis N, Jamaluddin AKM, Dholabhai PD, Bishnoi PR (1993, March) Effect of surfactants on hydrate formation kinetics. In SPE international symposium on oilfield chemistry. OnePetro
Daimaru T, Yamasaki A, Yanagisawa Y (2007) Effect of surfactant carbon chain length on hydrate formation kinetics. J Pet Sci Eng 56(1–3):89–96
Okutani K, Kuwabara Y, Mori YH (2008) Surfactant effects on hydrate formation in an unstirred gas/liquid system: an experimental study using methane and sodium alkyl sulfates. Chem Eng Sci 63:183–194
Ando N, Kodama T, Kondo W, Mori YH (2012) Clathrate hydrate formation from a methane+ ethane+ propane mixture in an unstirred surfactant-containing system. Energy Fuels 26(3):1798–1804
Wang F, Jia ZZ, Luo SJ, Fu SF, Wang L, Shi XS, Wang CS, Guo RB (2015) Effects of different anionic surfactants on methane hydrate formation. Chem Eng Sci 137:896–903
Dicharry C, Diaz J, Torré JP, Ricaurte M (2016) Influence of the carbon chain length of a sulfate-based surfactant on the formation of CO2, CH4 and CO2–CH4 gas hydrates. Chem Eng Sci 152:736–745
Link DD, Ladner EP, Elsen HA, Taylor CE (2003) Formation and dissociation studies for optimizing the uptake of methane by methane hydrates. Fluid Phase Equilib 211(1):1–10
Di Profio P, Arca S, Germani R, Savelli G (2007) Novel nanostructured media for gas storage and transport: clathrate hydrates of methane and hydrogen. J Fuel Cell Sci Technol. https://doi.org/10.1115/1.2393304
Fazlali A, Kazemi SA, Keshavarz-Moraveji M, Mohammadi AH (2013) Impact of different surfactants and their mixtures on methane-hydrate formation. Energy Technol 1(8):471–477
Du J, Li H, Wang L (2014) Effects of ionic surfactants on methane hydrate formation kinetics in a static system. Adv Powder Technol 25(4):1227–1233
Saw VK, Gudala M, Udayabhanu G, Mandal A, Laik S (2014) Kinetics of methane hydrate formation and its dissociation in presence of non-ionic surfactant Tergitol. J Unconv Oil Gas Resour 6:54–59
Sa JH, Lee BR, Park DH, Han K, Chun HD, Lee KH (2011) Amino acids as natural inhibitors for hydrate formation in CO2 sequestration. Environ Sci Technol 45(13):5885–5891
Sa JH, Kwak GH, Han K, Ahn D, Cho SJ, Lee JD, Lee KH (2016) Inhibition of methane and natural gas hydrate formation by altering the structure of water with amino acids. Sci Rep 6(1):1–9
Prasad PS, Kiran BS (2018) Are the amino acids thermodynamic inhibitors or kinetic promoters for carbon dioxide hydrates? J Nat Gas Sci Eng 52:461–466
Bavoh CB, Lal B, Osei H, Sabil KM, Mukhtar H (2019) A review on the role of amino acids in gas hydrate inhibition, CO2 capture and sequestration, and natural gas storage. J Nat Gas Sci Eng 64:52–71
Mohammad-Taheri M, Moghaddam AZ, Nazari K, Zanjani NG (2012) Methane hydrate stability in the presence of water-soluble hydroxyalkyl cellulose. J Nat Gas Chem 21(2):119–125
Al-Adel S, Dick JA, El-Ghafari R, Servio P (2008) The effect of biological and polymeric inhibitors on methane gas hydrate growth kinetics. Fluid Phase Equilib 267(1):92–98
Karaaslan U, Parlaktuna M (2002) Promotion effect of polymers and surfactants on hydrate formation rate. Energy Fuels 16(6):1413–1416
Fakharian H, Ganji H, Far AN, Kameli M (2012) Potato starch as methane hydrate promoter. Fuel 94:356–360
Ganji H, Manteghian M, Mofrad HR (2007) Effect of mixed compounds on methane hydrate formation and dissociation rates and storage capacity. Fuel Process Technol 88(9):891–895
Babakhani SM, Alamdari A (2015) Effect of maize starch on methane hydrate formation/dissociation rates and stability. J Nat Gas Sci Eng 26:1–5
Longinos S (2020) The effect of experimental process on natural gas hydrate formation, PhD Thesis, petroleum & natural gas engineering department, Middle East Technical University, Ankara, Turkey
Longinos SN, Parlaktuna M (2021) Are the amino acids inhibitors or promoters on methane (95%) – propane (5%) hydrate formation? React Kinet Mech Cat 132(2):771–794. https://doi.org/10.1007/s11144-021-01959-0
Longinos SN, Parlaktuna M (2021) Examination of behavior of lysine on methane (95%) – propane (5%) hydrate formation by the use of different impellers. J Pet Explor Prod Technol 11(4):1823–1831. https://doi.org/10.1007/s13202-021-01146-w
Longinos SN, Parlaktuna M (2021) Kinetic analysis of arginine, glycine and valine on methane (95%) – propane (5%) hydrate formation. React Kinet Mech Cat 133(2):741–751. https://doi.org/10.1007/s11144-021-02018-4
Longinos SN, Parlaktuna M (2021) Kinetic study of amino acids on methane (95%) – propane (5%) hydrate formation. React Kinet Mech Cat 133(2):753–763. https://doi.org/10.1007/s11144-021-02023-7
Longinos SN, Parlaktuna M (2021) Examination of asparagine, aspartic acid and threonine in methane (95%)-propane (5%) gas hydrates as kinetic inhibitors. React Kinet Mech Cat. https://doi.org/10.1007/s11144-021-02052-2
Lee BI, Kesler MG (1975) A generalized thermodynamic correlation based on three-parameter corresponding states. AIChE J 21(3):510–527
Cai Y, Chen Y, Li Q, Li L, Huang H, Wang S, Wang W (2017) CO2 hydrate formation promoted by a natural amino acid l-methionine for possible application to CO2 capture and storage. Energy Technol 5(8):1195–1199
Veluswamy HP, Lee PY, Premasinghe K, Linga P (2017) Effect of biofriendly amino acids on the kinetics of methane hydrate formation and dissociation. Ind Eng Chem Res 56(21):6145–6154
Maddah M, Maddah M, Peyvandi K (2018) Molecular dynamics simulation of methane hydrate formation in presence and absence of amino acid inhibitors. J Mol Liq 269:721–732
Liu Y, Chen B, Chen Y, Zhang S, Guo W, Cai Y, Tan B, Wang W (2015) Methane storage in a hydrated form as promoted by leucines for possible application to natural gas transportation and storage. Energy Technol 3(8):815–819
Longinos SN, Parlaktuna M (2020) The effect of experimental conditions on methane (95%)–propane (5%) hydrate formation. Energies 13(24):6710
Longinos SN, Parlaktuna M (2021) Kinetic analysis of methane–propane hydrate formation by the use of different impellers. ACS Omega 6(2):1636–1646
Longinos SN, Parlaktuna M (2021) Kinetic analysis of dual impellers on methane hydrate formation. Int J Chem React Eng 19(2):155–165
Misyura SY, Donskoy IG (2020) Dissociation kinetics of methane hydrate and CO2 hydrate for different granular composition. Fuel 262:116614
Longinos SN, Parlaktuna M (2021) The effect of experimental conditions on methane hydrate formation by the use of single and dual impellers. React Kinet Mech Cat 132:771–794
Longinos SN, Parlaktuna M (2021) Kinetic analysis of CO2 hydrate formation by the use of different impellers. React Kinet Mech Cat 133:85–100
Longinos SN, Parlaktuna M (2021) Examination of methane hydrate formation by the use of dual impeller combinations. React Kinet Mech Cat 133(2):729–740. https://doi.org/10.1007/s11144-021-02017-5
Longinos SN, Longinou DD, Celebi E, Toktarbay Z, Parlaktuna M (2021) Kinetic study of methane hydrate formation with the use of surface baffle. React Kinet Mech Cat. https://doi.org/10.1007/s11144-021-02058-w
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Longinos, S.N., Longinou, DD., Parlaktuna, M. et al. The impact of methionine, tryptophan and proline on methane (95%)–propane (5%) hydrate formation. Reac Kinet Mech Cat 134, 653–664 (2021). https://doi.org/10.1007/s11144-021-02089-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-02089-3