Abstract
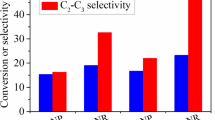
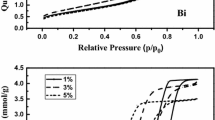
Y2O3 nanorods were prepared via a hydrothermal method. A series of Sr-modified Y2O3 nanorods (Sr–Y2O3–NR) with a Sr/Y molar ratio of 0.02–0.06 were synthesized by an impregnation method, and studied with respect to their performance in the oxidative coupling of methane (OCM). The structural and physicochemical properties of these catalysts were characterized by means of XRD, N2 adsorption, SEM, TEM, XPS, O2-TPD and CO2-TPD. Y2O3 nanorods exhibit higher CH4 conversion and C2–C3 selectivity relative to Y2O3 nanoparticles, which could link with the fact that Y2O3 nanorods predominantly expose (440) and (222) planes. The addition of a small amount of Sr to Y2O3 nanorods enhances the activation of oxygen, the ratio of (O− + O2−)/O2− and amount of moderate basic sites for the Sr–Y2O3-NR catalysts, thus promoting the OCM performance. The best 0.04Sr–Y2O3-NR catalyst with a Sr/Y molar ratio of 0.04 can give a 23.0% CH4 conversion with 50.2% C2–C3 selectivity at 650 °C. We found that the C2–C3 yield achieved on the Y2O3-based catalysts correlated well with the amount of moderate basic sites present on the catalysts.








Similar content being viewed by others
References
Lee J, Oyama S (1988) Oxidative coupling of methane to higher hydrocarbons. Catal Rev Sci Eng 30(2):249–280
Arndt S, Laugel G, Levchenko S, Horn R, Baerns M, Scheffler M, Schlögl R, Schomäcker R (2011) A critical assessment of Li/MgO-based catalysts for the oxidative coupling of methane. Catal Rev Sci Eng 53(4):424–514
Ge XM, Yang LC, Sheets JP, Yu ZT, Li YB (2014) Biological conversion of methane to liquid fuels: status and opportunities. Biotechnol Adv 32(8):1460–1475
Tang P, Zhu QJ, Wu ZX, Ma D (2014) Methane activation: the past and future. Energy Environ Sci 7(8):2580–2591
Taifan W, Baltrusaitis J (2016) CH4 conversion to value added products: potential, limitations and extensions of a single step heterogeneous catalysis. Appl Catal B 198:525–547
Zakaria Z, Kamarudin SK (2016) Direct conversion technologies of methane to methanol: an overview. Renew Sust Energ Rev 65:250–261
Galadima A, Muraza O (2016) Revisiting the oxidative coupling of methane to ethylene in the golden period of shale gas: a review. J Ind Eng Chem 37:1–13
Han B, Yang Y, Xu Y, Etim UJ, Qiao K, Xu B, Yan Z (2016) A review of the direct oxidation of methane to methanol. Chin J Catal 37(8):1206–1215
Schwach P, Pan XL, Bao XH (2017) Direct conversion of methane to value-added chemicals over heterogeneous catalysts: challenges and prospects. Chem Rev 117(13):8497–8520
Gambo Y, Jalil AA, Triwahyono S, Abdulrasheed AA (2018) Recent advances and future prospect in catalysts for oxidative coupling of methane to ethylene: a review. J Ind Eng Chem 59:218–229
Zhao GY, Drewery M, Mackie J, Oliver T, Kennedy EM, Stockenhuber M (2020) The catalyzed conversion of methane to value-added products. Energy Technol 8(8):1900665
Sun LL, Wang Y, Guan NJ, Li LD (2020) Methane activation and utilization: current status and future challenges. Energy Technol 8(8):1900826
Arinaga AM, Ziegelski MC, Marks TJ (2021) Alternative oxidants for the catalytic oxidative coupling of methane. Angew Chem Int Ed 60(19):10502–10514
Keller GE, Bhasin MM (1982) Synthesis of ethylene via oxidative coupling of methane I Determination of active catalysts. J Catal 73(1):9–19
Ren T, Patel M, Kornelis B (2006) Olefins from conventional and heavy feedstocks: energy use in steam cracking and alternative processes. Energy 31(4):425–451
Huang P, Zhao YH, Zhang J, Zhu Y, Sun YH (2013) Exploiting shape effects of La2O3 nanocatalysts for oxidative coupling of methane reaction. Nanoscale 5(22):10844–10848
Jiang T, Song JJ, Huo MF, Yang NT, Liu JW, Zhang J, Sun YH, Zhu Y (2016) La2O3 catalysts with diverse spatial dimensionality for oxidative coupling of methane to produce ethylene and ethane. RSC Adv 6(41):34872–34876
Fu B, Jiang T, Zhu Y (2018) Structural effect of one-dimensional samarium oxide catalysts on oxidative coupling of methane. J Nanosci Nanotechnol 18(5):3398–3404
Sun YN, Shen Y, Song JJ, Ba RB, Huang SS, Zhao YH, Zhang J, Sun YH, Zhu Y (2016) Facet-controlled CeO2 nanocrystals for oxidative coupling of methane. J Nanosci Nanotechnol 16(5):4692–4700
Takenaka S, Kaburagi T, Yamanaka I, Otsuka K (2001) Oxidative coupling of methane over Li+-added Y2O3 catalyst prepared from Y(OH)3. Catal Today 71(1–2):31–36
Papa F, Luminita P, Osiceanu P, Birjega R, Akane M, Balint I (2011) Acid-base properties of the active sites responsible for C2+ and CO2 formation over MO–Sm2O3 (M = Zn, Mg, Ca and Sr) mixed oxides in OCM reaction. J Mol Catal A 346(1–2):46–54
Song JJ, Sun YN, Ba RB, Huang SS, Zhao YH, Sun YH, Zhu Y (2015) Monodisperse Sr–La2O3 hybrid nanofibers for oxidative coupling of methane to synthesize C2 hydrocarbons. Nanoscale 7(6):2260–2264
Ferreira VJ, Tavares P, Figueiredo JL, Faria JL (2012) Effect of Mg, Ca, and Sr on CeO2 based catalysts for the oxidative coupling of methane: Investigation on the oxygen species responsible for catalytic performance. Ind Eng Chem Res 51(32):10535–10541
Fan YQ, Sun MX, Miao CX, Yue YH, Hua WM, Gao Z (2021) Morphology effects of nanoscale Er2O3 and Sr-Er2O3 catalysts for oxidative coupling of methane. Catal Lett 151(8):2197–2206
Fan YQ, Miao CX, Yue YH, Hua WM, Gao Z (2021) Nanosheet-like Ho2O3 and Sr-Ho2O3 catalysts for oxidative coupling of methane. Catalysts 11(3):388
Haneda M, Tanaka M, Doi Y, Bion N (2018) Oxidative coupling of methane over Ba-doped Y2O3 catalyst-Similarity with active site for direct decomposition of NO. Mol Catal 457:74−81
Long RQ, Wan HL (1997) Oxidative coupling of methane over SrF2/Y2O3 catalyst. Appl Catal A 159(1–2):45–58
Heneda M, Katsuragawa Y, Nakamura Y, Towata A (2018) Promoting effect of cerium oxide on the catalytic performance of yttrium oxide for oxidative coupling of methane. Front Chem 6:581
Zhao MQ, Ke SC, Wu HQ, Xia WS, Wan HL (2019) Flower-like Sr-La2O3 microspheres with hierarchically porous structures for oxidative coupling of methane. Ind Eng Chem Res 58(51):22847–22856
Kharas KCC, Lunsford JH (1989) Catalytic partial oxidation of methane over barium metaplumbate BaPbO3: possible involvement of peroxide ion. J Am Chem Soc 111(6):2336–2337
Peng XD, Richards DA, Stair PC (1990) Surface composition and reactivity of lithium-doped magnesium oxide catalysts for oxidative coupling of methane. J Catal 121(1):99–109
Ding WP, Chen Y, Fu XC (1994) Oxidative coupling of methane over Ce4+-doped Ba3WO6 catalysts: investigation on oxygen species responsible for catalytic performance. Catal Lett 23(1–2):69–78
Hou YH, Han WC, Xia WS, Wan HL (2015) Structure sensitivity of La2O2CO3 catalysts in the oxidative coupling of methane. ACS Catal 5(3):1663–1674
Bai Y, Xia WS, Weng WZ, Lian MS, Zhao MQ, Wan HL (2018) Influence of phosphate on La-based catalysts for oxidative coupling of methane. Chem J Chin Univ Chin 39(2):247–254
Sayle TXT, Parker SC, Sayle DC (2005) Oxidising CO to CO2 using ceria nanoparticles. Phys Chem Chem Phys 7(15):2936–2941
Spinicci R, Tofanari A (1990) Characterization of catalysts for methane-coupling by means of temperature programmed desorption. Catal Today 6(4):473–479
Xu J, Zhang Y, Xu X, Fang X, Xi R, Liu Y, Zheng R, Wang X (2019) Constructing La2B2O7 (B = Ti, Zr, Ce) compounds with three typical crystalline phases for the oxidative coupling of methane: The effect of phase structures, superoxide anions, and alkalinity on the reactivity. ACS Catal 9(5):4030–4045
McFarland EW, Metiu H (2013) Catalysis by doped oxides. Chem Rev 113(6):4391–4427
Liang Q, Wu X, Weng D, Xu H (2008) Oxygen activation on Cu/Mn−Ce mixed oxides and the role in diesel soot oxidation. Catal Today 139(1–2):113–118
Elkins TW, Roberts SJ, Hagelin-Weaver HE (2016) Effects of alkali and alkaline-earth metal dopants on magnesium oxide supported rare-earth oxide catalysts in the oxidative coupling methane. Appl Catal A 528:175–190
Driscoll DJ, Martir W, Wang JX, Lunsford JH (1985) Formation of gas-phase methyl radicals over MgO. J Am Chem Soc 107(1):58–63
Bernal S, Blanco G, El Amarti A, Cifredo G, Fitian L, Galtayries A, Martín J, Pintado JM (2006) Surface basicity of ceria-supported lanthana. Influence of the calcination temperature. Surf Interface Anal 38(4):229–233
Peng L, Xu J, Fang X, Liu W, Xu X, Liu L, Li Z, Peng H, Zheng R, Wang X (2018) SnO2 based catalysts with low-temperature performance for oxidative coupling of methane: Insight into the promotional effects of alkali-metal oxides. Eur J Inorg Chem 17:1787–1799
Xu J, Peng L, Fang X, Fu Z, Liu W, Xu X, Peng H, Zheng R, Wang X (2018) Developing reactive catalysts for low temperature oxidative coupling of methane: on the factors deciding the reaction performance of Ln2Ce2O7 with different rare earth A sites. Appl Catal A 552:117–128
Xu J, Zhang Y, Liu Y, Fang X, Xu X, Liu W, Zheng R, Wang X (2019) Optimizing the reaction performance of La2Ce2O7-based catalysts for oxidative coupling of methane (OCM) at lower temperature by lattice doping with Ca cations. Eur J Inorg Chem 2:183–194
Wang Z, Zou G, Luo X, Liu H, Gao R, Chou L, Wang X (2012) Oxidative coupling of methane over BaCl2-TiO2-SnO2 catalyst. J Nat Gas Chem 21(1):49–55
Cheng F, Yang J, Yan L, Zhao J, Zhao HH, Song HL, Chou LJ (2018) Impact of chloride ions on the oxidative coupling of methane over Li/SnO2 catalyst. React Kinet Mech Catal 125(2):675–688
Bernal S, Botana FJ, Garcia R, Rodiguez-Izquierdo JM (1987) Behaviour of rare earth sesquioxides exposed to atmospheric carbon dioxide and water. React Soliak 4(1–2):23–40
Djerdj I, Garnweitner G, Su DS, Niederberger M (2007) Morphology-controlled nonaqueous synthesis of anisotropic lanthanum hydroxide nanoparticles. J Solid State Chem 180(7):2154–2165
Farrukh MA, Imran F, Ali S, Khaleeq-ur-Rahman M, Naqvi II (2015) Micelle assisted synthesis of La2O3 nanoparticles and their applications in photodegradation of bromophenol blue. Russ J Appl Chem 88(9):1523–1527
Ito T, Wang JX, Lin CH, Lunsford JH (1985) Oxidative dimerization of methane over a lithium-promoted magnesium oxide catalyst. J Am Chem Soc 107(18):5062–5068
Yamashita H, Machida Y, Tomita A (1991) Oxidative coupling of methane with peroxide ions over barium-lanthanum-oxygen mixed oxide. Appl Catal A 79(2):203–214
Sollier BM, Bonne M, Khenoussi N, Michelin L, Miró EE, Gómez LE, Boix AV, Lebeau B (2020) Synthesis and characterization of electrospun nanofibers of Sr-La-Ce oxides as catalysts for the oxidative coupling of methane. Ind Eng Chem Res 59(25):11419–11430
Acknowledgements
Financial support of this work was provided by the National Key R&D Program of China (No. 2017YFB0602200), the National Natural Science Foundation of China (No. 91645201), the Science and Technology Commission of Shanghai Municipality (No. 19DZ2270100), and the Shanghai Research Institute of Petrochemical Technology SINOPEC (No. 33750000-19-ZC0607-0005).
Author information
Authors and Affiliations
Contributions
WH, CM: Conceptualization; WH, YY: Methodology; YF: Formal analysis and investigation; YF: Writing—original draft preparation; WH, ZG: Writing—review and editing; WH, CM:Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Data availability
The datasets of current study are available from the corresponding authors on reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fan, Y., Miao, C., Yue, Y. et al. Oxidative coupling of methane over Y2O3 and Sr–Y2O3 nanorods. Reac Kinet Mech Cat 134, 711–725 (2021). https://doi.org/10.1007/s11144-021-02085-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-02085-7