Abstract
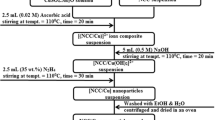
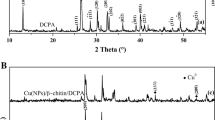
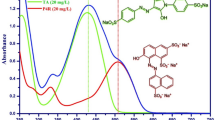
Herein we report the performance of cheaper, more efficient and eco-friendlier chitin (CN) and chitosan (CS) biopolymers supported Cu nanoparticles (Cu NPs) catalysts (1.5 wt% Cu/CN) and (4.5 wt% Cu/CS) in the reaction model of para-nitrophenol (p-NP) reduction to para-aminophenol (p-AP) by NaBH4. The catalysts were synthetized with impregnation method and CN was extracted from local shrimp shells wastes, while CS was obtained by the deacetylation of CN. It was found that the activity of 1.5 wt% Cu/CN, with a lower Cu loading, is better than that of 4.5 wt% Cu/CS, which achieved 100% p-NP conversion to p-AP in short reaction times at all studied reaction temperatures. The activity of each catalyst was found to depend on the interaction modes of Cu NPs with the functional groups of CN and CS, which affects the textural parameters of the catalysts and the dispersion of Cu NPs, as revealed by various characterization techniques used. Kinetic of p-NP reduction was found to follows the pseudo-first order with respect to p-NP concentration. The apparent rate constants at T = 25 °C were calculated to be kapp = 0.854 min−1 and 0.350 min−1 for 1.5 wt% Cu/CN and 4.5 wt% Cu/CS catalysts, respectively, which increased with the reaction temperature. Kinetics data of p-NP reduction at T = 25 °C, obtained for various concentrations of reagents, were successfully modeled using the Langmuir–Hinshelwood mechanism. The related kinetic parameters such as the adsorption equilibrium constants K(p-NP), K(\({\text{BH}}_{4}^{ - }\)) and the surface rate constant, k, were calculated. The competitive adsorption between p-NP and \({\text{BH}}_{4}^{ - }\) was shown to control the rate of p-NP reduction to p-AP.






Similar content being viewed by others
References
González JA, Villanueva ME, Piehl LL, Copello GJ (2015) Development of a chitin/graphene oxide hybrid composite for the removal of pollutant dyes: adsorption and desorption study. Chem Eng J 280:41–48. https://doi.org/10.1016/j.cej.2015.05.112
Auta M, Hameed BH (2013) Coalesced chitosan activated carbon composite for batch and fixed-bed adsorption of cationic and anionic dyes. Colloids Surf B 105:199–206. https://doi.org/10.1016/j.colsurfb.2012.12.021
Ahmad N, Sultana S, Khan MZ, Sabir S (2020) Chitosan based nanocomposites as efficient adsorbents for water treatment. Mod Age Waste Water Probl. https://doi.org/10.1007/978-3-030-08283-3_4
Shajahan A, Shankar S, Sathiyaseelan A et al (2017) Comparative studies of chitosan and its nanoparticles for the adsorption efficiency of various dyes. Int J Biol Macromol 104:1449–1458. https://doi.org/10.1016/j.ijbiomac.2017.05.128
Liu Y, Li L, Duan Z et al (2021) Chitosan modified nitrogen-doped porous carbon composite as a highly-efficient adsorbent for phenolic pollutants removal. Colloids Surf A 610:125728. https://doi.org/10.1016/j.colsurfa.2020.125728
Dandil S, Akin Sahbaz D, Acikgoz C (2019) Adsorption of Cu(II) ions onto crosslinked chitosan/Waste Active Sludge Char (WASC) beads: kinetic, equilibrium, and thermodynamic study. Int J Biol Macromol 136:668–675. https://doi.org/10.1016/j.ijbiomac.2019.06.063
González JA, Bafico JG, Villanueva ME et al (2018) Continuous flow adsorption of ciprofloxacin by using a nanostructured chitin/graphene oxide hybrid material. Carbohydr Polym 188:213–220. https://doi.org/10.1016/j.carbpol.2018.02.021
Molnár Á (2019) The use of chitosan-based metal catalysts in organic transformations. Coord Chem Rev 388:126–171. https://doi.org/10.1016/j.ccr.2019.02.018
Gates WP, MacLeod AJ, Fehervari A et al (2020) Interactions of per- and polyfluoralkyl substances (PFAS) with landfill liners. Adv Environ Eng Res. https://doi.org/10.21926/aeer.2004007
Anusuya N, Pragathiswaran C, Thulasi G (2020) Catalytic reduction of 4-nitrophenol to 4-amino phenol by chitosan/TiO2-Fe2O3 nanomaterial. Mater Today Proc 37:3759–3763. https://doi.org/10.1016/j.matpr.2020.10.570
Kavitha T, Kumar S, Prasad V et al (2019) Nio powder synthesized through nickel metal complex degradation for water treatment. Desalin Water Treat 155:216–224. https://doi.org/10.5004/dwt.2019.24054
Wang Y, Li Y, Liu S, Li B (2015) Fabrication of chitin microspheres and their multipurpose application as catalyst support and adsorbent. Carbohydr Polym 120:53–59. https://doi.org/10.1016/j.carbpol.2014.12.005
Ahlafi H, Moussout H, Boukhlifi F et al (2013) Kinetics of N-deacetylation of chitin extracted from shrimp shells collected from coastal area of Morocco. Mediterr J Chem 2:503–513. https://doi.org/10.13171/mjc.2.3.2013.22.01.20
Aazza M, Ahlafi H, Moussout H et al (2020) Catalytic reduction of nitro-phenolic compounds over Ag, Ni and Co nanoparticles catalysts supported on γ-Al2O3. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2020.103707
Ma X, Wu G, Dai F et al (2021) Chitosan/polydopamine layer by layer self-assembled silk fibroin nanofibers for biomedical applications. Carbohydr Polym 251:117058. https://doi.org/10.1016/j.carbpol.2020.117058
Yu S, Xu X, Feng J et al (2019) Chitosan and chitosan coating nanoparticles for the treatment of brain disease. Int J Pharm 560:282–293. https://doi.org/10.1016/j.ijpharm.2019.02.012
Chen X, Xu XJ, Zheng XC et al (2018) Chitosan supported palladium nanoparticles: the novel catalysts for hydrogen generation from hydrolysis of ammonia borane. Mater Res Bull 103:89–95. https://doi.org/10.1016/j.materresbull.2018.03.013
Ma F, Li P, Zhang B, Wang Z (2017) The facile synthesis of a chitosan Cu(II) complex by solution plasma process and evaluation of their antioxidant activities. Int J Biol Macromol 103:501–507. https://doi.org/10.1016/j.ijbiomac.2017.04.082
Reyes-Mercado E, Rivas-Loaiza JA, García-Merinos JP et al (2021) Chitosan-supported copper salt and copper metal nanoparticles/copper (I) oxide microcrystals: efficient and recyclable heterogeneous catalysts for the synthesis of bis(indolyl)methanes. Chem Eng Process Process Intensif. https://doi.org/10.1016/j.cep.2020.108201
Gritsch L, Lovell C, Goldmann WH, Boccaccini AR (2018) Fabrication and characterization of copper(II)-chitosan complexes as antibiotic-free antibacterial biomaterial. Carbohydr Polym 179:370–378. https://doi.org/10.1016/j.carbpol.2017.09.095
Akhtar MA, Ilyas K, Dlouhý I et al (2020) Electrophoretic deposition of copper(II)-chitosan complexes for antibacterial coatings. Int J Mol Sci. https://doi.org/10.3390/ijms21072637
Zhang Y, Xue C, Xue Y et al (2005) Determination of the degree of deacetylation of chitin and chitosan by X-ray powder diffraction. Carbohydr Res 340:1914–1917. https://doi.org/10.1016/j.carres.2005.05.005
Hao G, Hu Y, Shi L et al (2021) Physicochemical characteristics of chitosan from swimming crab (Portunus trituberculatus) shells prepared by subcritical water pretreatment. Sci Rep 11:1–9. https://doi.org/10.1038/s41598-021-81318-0
Ali F, Khan SB, Kamal T et al (2018) Synthesis and characterization of metal nanoparticles templated chitosan-SiO2 catalyst for the reduction of nitrophenols and dyes. Carbohydr Polym 192:217–230. https://doi.org/10.1016/j.carbpol.2018.03.029
Ali F, Khan SB, Kamal T et al (2017) Anti-bacterial chitosan/zinc phthalocyanine fibers supported metallic and bimetallic nanoparticles for the removal of organic pollutants. Carbohydr Polym 173:676–689. https://doi.org/10.1016/j.carbpol.2017.05.074
Zayed MF, Eisa WH, Hosam AEHM, Abou Zeid AM (2020) Spectroscopic investigation of chitosan-supported Cu2O/CuO nanocomposite; a separable catalyst for water-pollutants degradation. J Alloys Compd 835:155306. https://doi.org/10.1016/j.jallcom.2020.155306
Cai Y, Zheng L, Fang Z (2015) Selective adsorption of Cu(II) from an aqueous solution by ion imprinted magnetic chitosan microspheres prepared from steel pickling waste liquor. RSC Adv 5:97435–97445. https://doi.org/10.1039/c5ra16547d
Bakhsh EM, Ali F, Khan SB et al (2019) Copper nanoparticles embedded chitosan for efficient detection and reduction of nitroaniline. Int J Biol Macromol 131:666–675. https://doi.org/10.1016/j.ijbiomac.2019.03.095
de Souza JF, da Silva GT, Fajardo AR (2017) Chitosan-based film supported copper nanoparticles: a potential and reusable catalyst for the reduction of aromatic nitro compounds. Carbohydr Polym 161:187–196. https://doi.org/10.1016/j.carbpol.2017.01.018
Kassem AA, Abdelhamid HN, Fouad DM, Ibrahim SA (2021) Catalytic reduction of 4-nitrophenol using copper terephthalate frameworks and CuO@C composite. J Environ Chem Eng 9:104401. https://doi.org/10.1016/j.jece.2020.104401
Fu Y, Huang T, Jia B et al (2017) Reduction of nitrophenols to aminophenols under concerted catalysis by Au/g-C3N4 contact system. Appl Catal B 202:430–437. https://doi.org/10.1016/j.apcatb.2016.09.051
Kamal MS, Razzak SA, Hossain MM (2016) Catalytic oxidation of volatile organic compounds (VOCs)—a review. Atmos Environ 140:117–134. https://doi.org/10.1016/j.atmosenv.2016.05.031
Khan MSJ, Kamal T, Ali F et al (2019) Chitosan-coated polyurethane sponge supported metal nanoparticles for catalytic reduction of organic pollutants. Int J Biol Macromol 132:772–783. https://doi.org/10.1016/j.ijbiomac.2019.03.205
Verma AD, Mandal RK, Sinha I (2015) Kinetics of p-nitrophenol reduction catalyzed by PVP stabilized copper nanoparticles. Catal Lett 145:1885–1892. https://doi.org/10.1007/s10562-015-1605-5
Wunder S, Polzer F, Lu Y et al (2010) Kinetic analysis of catalytic reduction of 4-nitrophenol by metallic nanoparticles immobilized in spherical polyelectrolyte brushes. J Phys Chem C 114:8814–8820. https://doi.org/10.1021/jp101125j
Kästner C, Thünemann AF (2016) Catalytic reduction of 4-nitrophenol using silver nanoparticles with adjustable activity. Langmuir 32:7383–7391. https://doi.org/10.1021/acs.langmuir.6b01477
Dang TMD, Le TTT, Fribourg-Blanc E, Dang MC (2011) Synthesis and optical properties of copper nanoparticles prepared by a chemical reduction method. Adv Nat Sci Nanosci Nanotechnol. https://doi.org/10.1088/2043-6262/2/1/015009
Guibal E (2005) Heterogeneous catalysis on chitosan-based materials: a review. Prog Polym Sci 30:71–109. https://doi.org/10.1016/j.progpolymsci.2004.12.001
Narayanan KB, Sakthivel N (2011) Synthesis and characterization of nano-gold composite using Cylindrocladium floridanum and its heterogeneous catalysis in the degradation of 4-nitrophenol. J Hazard Mater 189:519–525. https://doi.org/10.1016/j.jhazmat.2011.02.069
Usman MS, Ibrahim NA, Shameli K et al (2012) Copper nanoparticles mediated by chitosan: synthesis and characterization via chemical methods. Molecules 17:14928–14936. https://doi.org/10.3390/molecules171214928
Anbinder PS, Macchi C, Amalvy J, Somoza A (2019) A study of the structural changes in a chitosan matrix produced by the adsorption of copper and chromium ions. Carbohydr Polym 222:114987. https://doi.org/10.1016/j.carbpol.2019.114987
Hervés P, Pérez-Lorenzo M, Liz-Marzán LM et al (2012) Catalysis by metallic nanoparticles in aqueous solution: model reactions. Chem Soc Rev 41:5577–5587. https://doi.org/10.1039/c2cs35029g
Bingwa N, Meijboom R (2014) Kinetic evaluation of dendrimer-encapsulated palladium nanoparticles in the 4-nitrophenol reduction reaction. J Phys Chem C 118:19849–19858. https://doi.org/10.1021/jp505571p
Wunder S, Lu Y, Albrecht M, Ballauff M (2011) Catalytic activity of faceted gold nanoparticles studied by a model reaction: evidence for substrate-induced surface restructuring. ACS Catal 1:908–916. https://doi.org/10.1021/cs200208a
Gu S, Lu Y, Kaiser J et al (2015) Kinetic analysis of the reduction of 4-nitrophenol catalyzed by Au/Pd nanoalloys immobilized in spherical polyelectrolyte brushes. Phys Chem Chem Phys 17:28137–28143. https://doi.org/10.1039/c5cp00519a
Lente G (2015) Deterministic kinetics in chemistry and systems biology: the dynamics of complex reaction networks. Springer, New York
Acknowledgements
This work was supported by MESRSFC and CNRST − Rabat- Morocco, within the framework of the PPR2 project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mounir, C., Ahlafi, H., Aazza, M. et al. Kinetics and Langmuir–Hinshelwood mechanism for the catalytic reduction of para-nitrophenol over Cu catalysts supported on chitin and chitosan biopolymers. Reac Kinet Mech Cat 134, 285–302 (2021). https://doi.org/10.1007/s11144-021-02066-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-02066-w