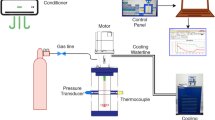
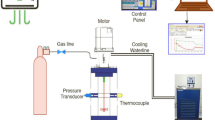
Abstract
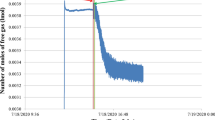
The motivation for this study was the potential of arginine, glycine and valine to be examined as promoter or inhibitor on methane (95%)–propane (5%) hydrate formation and the examination of which flow is better for hydrate formation. The experimental outcomes indicated that glycine can be used as inhibitor while valine and arginine can be used as promoters. Rushton turbine experiments had higher values in rates of hydrate formation compared to pitched blade turbines with percentage difference of 4.3% and 4.93% between RT vs PBTU and RT vs PBTD, showing that radial flow creates better conditions for hydrate formation.






Similar content being viewed by others
References
Sloan ED, Koh C (1998) Clathrate hydrates of natural gases. Mercel Dekker, New York
Ripmeester JA, John ST, Ratcliffe CI, Powell BM (1987) A new clathrate hydrate structure. Nature 325(6100):135–136
Longinos SN, Longinou DD, Achinas S (2020) Natural gas hydrates: possible environmental issues. Contemporary environmental issues and challenges in era of climate change. Springer, Singapore
Longinos S (2019) Potential environmental challenges for gas hydrates. LAP Lambert Academic Publishing, Beau Basin
Kvenvolden KA (2000) Natural gas hydrate: background and history of discovery. Natural gas hydrate. Springer, Dordrecht
Carson DB, Katz DL (1942) Natural gas hydrates. Trans AIME 146(01):150–158
Sun Y, Jiang S, Li S, Zhang G, Guo W (2019) Growth kinetics of hydrate formation from water–hydrocarbon system. Chin J Chem Eng 27(9):2164–2179
Meng Q, Liu H, Wang J (2017) A critical review on fundamental mechanisms of spontaneous imbibition and the impact of boundary condition, fluid viscosity and wettability. Adv Geo-Energy Res 1(1):1–17
Li SL, Sun CY, Liu B, Li ZY, Chen GJ, Sum AK (2014) New observations and insights into the morphology and growth kinetics of hydrate films. Sci Rep 4(1):1–6
Li SL, Sun CY, Liu B, Feng XJ, Li FG, Chen LT, Chen GJ (2013) Initial thickness measurements and insights into crystal growth of methane hydrate film. AIChE J 59(6):2145–2154
Partoon B, Wong NM, Sabil KM, Nasrifar K, Ahmad MR (2013) A study on thermodynamics effect of [EMIM]-Cl and [OH-C2MIM]-Cl on methane hydrate equilibrium line. Fluid Phase Equilib 337:26–31
Nashed O, Sabil KM, Lal B, Ismail L, Jaafar AJ (2014) Study of 1-(2-hydroxyethyle) 3-methylimidazolium halide as thermodynamic inhibitors. Appl Mech Mater 625:337–340
Xu CG, Li XS (2014) Research progress of hydrate-based CO2 separation and capture from gas mixtures. RSC Adv 4(35):18301–18316
Anderson FE, Prausnitz JM (1986) Inhibition of gas hydrates by methanol. AIChE J 32(8):1321–1333
Akhfash M, Arjmandi M, Aman ZM, Boxall JA, May EF (2017) Gas hydrate thermodynamic inhibition with MDEA for reduced MEG circulation. J Chem Eng Data 62(9):2578–2583
Kelland MA (2006) History of the development of low dosage hydrate inhibitors. Energy Fuels 20(3):825–847
Kvamme BB, Huseby G, Forrisdahl OK (1997) Molecular dynamics simulations of PVP kinetic inhibitor in liquid water and hydrate/liquid water systems. Mol Phys 90(6):979–992
Kvamme B, Kuznetsova T, Aasoldsen K (2005) Molecular dynamics simulations for selection of kinetic hydrate inhibitors. J Mol Graph Model 23(6):524–536
Ajiro H, Takemoto Y, Akashi M, Chua PC, Kelland MA (2010) Study of the kinetic hydrate inhibitor performance of a series of poly (N-alkyl-N-vinylacetamide) s. Energy Fuels 24(12):6400–6410
Villano LD, Kommedal R, Fijten MW, Schubert US, Hoogenboom R, Kelland MA (2009) A study of the kinetic hydrate inhibitor performance and seawater biodegradability of a series of poly (2-alkyl-2-oxazoline) s. Energy Fuels 23(7):3665–3673
Sa JH, Lee BR, Park DH, Han K, Chun HD, Lee KH (2011) Amino acids as natural inhibitors for hydrate formation in CO2 sequestration. Environ Sci Technol 45(13):5885–5891
Roosta H, Dashti A, Mazloumi SH, Varaminian F (2016) Inhibition properties of new amino acids for prevention of hydrate formation in carbon dioxide–water system: experimental and modeling investigations. J Mol Liq 215:656–663
Bavoh CB, Partoon B, Lal B, Gonfa G, Khor SF, Sharif AM (2017) Inhibition effect of amino acids on carbon dioxide hydrate. Chem Eng Sci 171:331–339
Prasad PS, Kiran BS (2018) Are the amino acids thermodynamic inhibitors or kinetic promoters for carbon dioxide hydrates? J Nat Gas Sci Eng 52:461–466
Bavoh CB, Nashed O, Khan MS, Partoon B, Lal B, Sharif AM (2018) The impact of amino acids on methane hydrate phase boundary and formation kinetics. J Chem Thermodyn 117:48–53
Rad SA, Khodaverdiloo KR, Karamoddin M, Varaminian F, Peyvandi K (2015) Kinetic study of amino acids inhibition potential of glycine and l-leucine on the ethane hydrate formation. J Nat Gas Sci Eng 26:819–826
Douïeb S, Fradette L, Bertrand F, Haut B (2015) Impact of the fluid flow conditions on the formation rate of carbon dioxide hydrates in a semi-batch stirred tank reactor. AIChE J 61(12):4387–4401
Longinos SN, Parlaktuna M (2020) The effect of experimental conditions on methane (95%)–propane (5%) hydrate formation. Energies 13(24):6710
Longinos SN, Parlaktuna M (2021) Kinetic analysis of CO2 hydrate formation by the use of different impellers. React Kinet Mech Catal. https://doi.org/10.1007/s11144-021-01968-z
Longinos SN, Parlaktuna M (2021) Examination of behavior of lysine on methane (95%)–propane (5%) hydrate formation by the use of different impellers. J Pet Explor Prod 11(4):1823–1831
Longinos SN, Parlaktuna M (2021) Are the amino acids inhibitors or promoters on methane (95%)–propane (5%) hydrate formation? React Kinet Mech Catal 132(2):795–809
Sankari ES, Manimegalai D (2017) Predicting membrane protein types using various decision tree classifiers based on various modes of general PseAAC for imbalanced datasets. J Theor Biol 435:208–217
Lee BI, Kesler MG (1975) A generalized thermodynamic correlation based on three-parameter corresponding states. AIChE J 21(3):510–527
Sa JH, Kwak GH, Lee BR, Park DH, Han K, Lee KH (2013) Hydrophobic amino acids as a new class of kinetic inhibitors for gas hydrate formation. Sci Rep 3(1):1–7
Partoon B, Malik SNA, Azemi MH, Sabil KM (2013) Experimental investigations on the potential of SDS as low-dosage promoter for carbon dioxide hydrate formation. Asia-Pac J Chem Eng 8(6):916–921
Sun T, Davies PL, Walker VK (2015) Structural basis for the inhibition of gas hydrates by α-helical antifreeze proteins. Biophys J 109(8):1698–1705
ZareNezhad B, Varaminian F (2013) A unified approach for description of gas hydrate formation kinetics in the presence of kinetic promoters in gas hydrate converters. Energy Convers Manage 73:144–149
Longinos SN, Parlaktuna M (2021) Kinetic analysis of dual impellers on methane hydrate formation. Int J Chem React Eng 19(2):155–165
Longinos SN, Parlaktuna M (2021) The effect of experimental conditions on methane hydrate formation by the use of single and dual impellers. React Kinet Mech Catal 132(2):771–794
Longinos SN, Parlaktuna M (2021) Kinetic analysis of methane–propane hydrate formation by the use of different impellers. ACS Omega 6(2):1636–1646
Vysniauskas A, Bishnoi PR (1983) A kinetic study of methane hydrate formation. Chem Eng Sci 38(7):1061–1072
Merey S, Longinos SN (2018) Investigation of gas seepages in Thessaloniki mud volcano in the Mediterranean Sea. J Pet Sci Eng 168:81–97. https://doi.org/10.1016/j.petrol.2018.05.014
Ribeiro CP, Lage PL (2008) Modelling of hydrate formation kinetics: state-of-the-art and future directions. Chem Eng Sci 63(8):2007–2034
Englezos P, Kalogerakis N, Dholabhai PD, Bishnoi PR (1987) Kinetics of formation of methane and ethane gas hydrates. Chem Eng Sci 42(11):2647–2658
Kamali R, Mansoorifar A, Manshadi MD (2014) Effect of baffle geometry on mixing performance in the passive micromixers. Iran J Sci Technol. Trans Mech Eng 38(M2):351
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Longinos, S.N., Parlaktuna, M. Kinetic analysis of arginine, glycine and valine on methane (95%)–propane (5%) hydrate formation. Reac Kinet Mech Cat 133, 741–751 (2021). https://doi.org/10.1007/s11144-021-02018-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-02018-4