Abstract
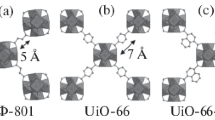
Porous Zr-based metal–organic frameworks (MOF-808 and MOF-808/H2SO4, UiO-66 and UiO-66-SO3H) were used as solid acid catalysts for N-formylation of aniline with formic acid (aniline/formic acid 1/2 mol/mol, 35 °C). The yield of N-formylaniline was demonstrated to depend on structure of active sites and decrease in the order: MOF-808/H2SO4 > MOF-808 > UiO-66-SO3H > UiO-66. The activity of Zr-MOFs was dependent on the key properties of solvent, i.e. polarity and basicity. The MOF-808/H2SO4 was found to be the best catalyst, resulting in the formation of N-formylaniline in 96% yield under solvent free conditions. Efficiencies of MOF-808 and MOF-808/H2SO4 were higher in comparison with H-ZSM-5, zeolite natrolite and zeolite heulandite.






Similar content being viewed by others
References
Dixneuf PH, Soulé JF (2019) Organometallics for green catalysis. Springer, Berlin
Kobayashi K, Nagato S, Kawakita M, Morikawa O, Konishi H (1995) Amberlite IR-120: a reusable catalyst for N-formylation of amines with formic acid using microwaves. Chem Lett 24:575–576
Chen BC, Bendarz MS, Zhao R, Sundeen JE, Chen P, Shen Z, Skoumbourdis AP, Barrish JC (2000) A new facile method for the synthesis of 1-arylimidazole-5-carboxylates. Tetrahedron Lett 41:5453–5456
Hartinez J, Laur J (1982) Active esters of formic acid as useful formylating agents: Improvements in the synthesis of formyl-amino acid esters, N-α-formyl-Met-Leu-Phe-OH, and formyl-Met-Lys-Pro-Arg, a phagocytosis stimulating peptide. Synthesis 11:979–981
Nasrollahzadeh M, Motahharifar N, Sajjadi M, Aghbolagh AM, Shokouhimehr M, Varma RS (2019) Recent advances in N-formylation of amines and nitroarenes using effcient (nano)catalysts in ecofriendly media. Green Chem 21:5144–5167
Blicke F, Lu CJ (1952) Formylation of amines with chloral and reduction of the N-formyl derivatives with lithium aluminum hydride. J Am Chem Soc 74:3933–3934
Reddy PG, Kumar GK, Baskaran S (2000) A convenient method for the N-formylation of secondary amines and anilines using ammonium formate. Tetrahedron Lett 41:9149–9151
Strazzolini P, Giumanini AG, Cauci S (1990) Acetic formic anhydride a review. Tetrahedron 46:1081–1118
Hill DR, Hsiao CN, Kurukulasuriya R, Wittenberger SJ (2002) 2,2,2-Trifluoroethyl formate: a versatile and selective reagent for the formylation of alcohols, amines, and N-hydroxylamines. Org Lett 4:111–113
Djuric SW (1984) a mild and convenient procedure for the N-formylation of secondary amines using organosilicon chemistry. J Org Chem 49:1311–1312
Krishnakumar B, Swaminathan M (2011) A convenient method for the N-formylation of amines at room temperature using TiO2-P25 or sulfated titania. J Mol Catal A: Chem 334:98–102
Chen FM, Benoiton NL (1979) A general method for formylating sensitive amino acid ester. Synth 9:709–710
Fieser LF, Jones JE (1995) Organic syntheses, collect, vol III. Wiley, New York
Rostamnia S, Karimi Z (2015) Preparation and catalytically study of metal–organic frameworks of amine/MIL-53(Al) as a powerful option in the rapid N-formylation condensation in neat conditions. Inorg Chim Acta 42:133–137
Jiang J, Yaghi OM (2015) Brønsted acidity in metal-organic frameworks. Chem Rev 115:6966–6997
Valenzano L, Civalleri B, Chavan S, Bordiga S, Nilsen MH, Jakobsen S, Lillerud KP, Lamberti C (2011) Disclosing the complex structure of UiO-66 metal organic framework: a synergic combination of experiment and theory. Chem Mater 23:1700–1718
Devautour-Vinot S, Maurin G, Serre C, Horcajada P, da Cunha DP, Guillerm V, de Souza Costa E, Taulelle F, Martineau C (2012) Structure and dynamics of the functionalized MOF type UiO-66(Zr): NMR and dielectric relaxation spectroscopies coupled with DFT calculations. Chem Mater 24:2168–2177
Fu G, Cirujano FG, Krajnc A, Mali G, Henrion M, Smolders S, De Vos DE (2020) Unexpected linker-dependent Brønsted acidity in the (Zr)UiO-66 metal organic framework and application to biomass valorization. Catal Sci Technol 10:4002–4009
Jiang J, Gandara F, Zhang YB, Na K, Yaghi OM, Klemperer WG (2014) Superacidity in sulfated metal-oganic framework-808. J Am Chem Soc 136:12844–12847
Qi Z, Chen L, Zhang S, Su J, Somorjai GA (2020) Integrating the fields of catalysis: active site engineering in metal cluster, metal organic framework and metal single Site. Top Catal 63:628–634
Liu P, Redekop E, Gao X, Liu WC, Olsbye U, Somorjai GA (2019) Oligomerization of light olefins catalyzed by Brønsted-acidic metal-organic framework-808. J Am Chem Soc 141:11557–11564
Ramirez-Caballero G, Ardila-Suarez C, Baldovino-Medrano V, (2018) (544as) Defect engineering and sulfation of MOF-808: towards the obtainment of microporous-mesoporous structures with strong Brønsted sites for catalysis applications, 18 AIChO Annual Meeting, Pittsburgh. https://www.aiche.org/conferences/aiche-annual-meeting/2018/proceeding/paper/
Jung SH, Ahn JH, Park SK, Choi JK (2002) A practical and convenient procedure for the N-formylation of amines using formic acid. Bull Korean Chem Soc 23:149–150
Jiang J, Gandara F, Zhang YB, Na K, Yaghi OM, Klempere WG (2014) Superacidity in sulfated metal-organic framework-808. J Am Chem Soc 136:12844–12847
Biswas S, Zhang J, Li Z, Liu YY, Grzywa M, Sun L, Volkmer D, Van Der Voort P (2013) Enhanced selectivity of CO2 over CH4 in sulphonate-, carboxylate-and iodo-functionalized UiO-66 frameworks. Dalton Trans 42:4730–4747
Gutmann V (1967) Empirical parameters for donor and acceptor properties of solvents. Electrochim Acta 2:661–670
Marcus Y (1991) The effectiveness of solvents as hydrogen bond donors. J Solut Chem 20:929–944
Reichardt C (1998) Solvents and solvent effects in organic chemistry. VCH, Weinheim
Babou F, Coudurier G, Vedrine JC (1995) Acidic properties of sulphated zirconia: an Infrared spectroscopic study. J Catal 152:341–349
Ardila-Suárez C, María D-L, Vaca LAD, Balbuena PB, Medrano VGB, Ramírez-Caballero GE (2019) Synthesis, characterization, and post-synthetic modification of a micro/mesoporous zirconium-tricarboxylate metal-organic framework: towards the addition of acid active sites. Cryst Eng Comm 21:3014–3030
Adeeva V, Dehaan JW, Janchen J, Lei GD, Schunemann V, Vandeven LJM, Sachtler WMH, Vansanten RA (1995) Acid sites in sulfated and metal-promoted zirconium dioxide catalysts. J Catal 151:364–372
Drago RS, Kob N (1997) Acidity and reactivity of sulfated zirconia and metal-doped sulfated zirconia. J Phys Chem B 101:3360–3364
Karimova NV, Luo M, Grassian VH, Benny Gerber R (2020) Absorption spectra of benzoic acid in water at different pH and in the presence of salts: insights from the integration of experimental data and theoretical cluster models. Chem Chem Phys 22:5046–5056
Kamath BV, Mehta JD, Bafna SL (2007) Ultraviolet absorption spectra: some substituted benzoic acids. J Appl Chem Biotechnol 25:743–751
Popova GY, Budneva AA, Andrushkevich TV (1997) Identification of adsorption forms by IR spectroscopy for formaldehyde and formic acid on K3PMo12O40. Reac Kinet Catal Lett 61:353–362
Mahalakshmi G, Balachandran V (2014) FT-IR and FT-Raman spectra, normal coordinate analysis and ab initio computations of trimesic acid. Spectrochim Acta Part A 124(535):547
Septawendar R, Nuruddin A, Sutardi S, Asri LATW, Maryani E, Setiawan AR, Purwasasmita BS (2019) Synthesis of one-dimensional ZrO2 nanomaterials from Zr(OH)4 precursors assisted by glycols through a facile precursor-templating method. Mater Res Express 6:065037
Getsoian A, Zhai Z, Bell AT (2014) Band-gap energy as a descriptor of catalytic activity for propene oxidation over mixed metal oxide catalysts. J Am Chem Soc 136:13684–13697
Mukherjee M, Bandyopadhyay B, Biswas P, Chakraborty T (2012) Amine inversion effects on the IR spectra of aniline in the gas phase and cold inert gas matrixes. Indian J Phys 86:201–208
Gee Ch, Douin S, Crepin C, Brechignac Ph (2001) Infrared spectroscopy of aniline (C6H5NH2) and its cation in cryogenic argon matrix. Chem Phys Lett 338:130–136
Moon SY, Liu Y, Hupp JT, Farha OK, Moon SY (2015) Instantaneous hydrolysis of nerve agent simulants with a six-connected zirconium based metal-organic framework. Angew Chem 127:6899–6903
Bhat SU, Naikoo RA, Tomar R, Bhat RA, Malla MA, Ganie JA, Kumar N, Rao TK (2017) Synthesis of N-formylation of amines using various ion exchanged forms of zeolite-a as catalysts. Pharm Pharmacol Int J 5:151–157
Bahari S, Mohammadi-Aghdam B, Sajadi SM, Zeidali F (2012) An efficient method for N-formylation of amines using natural HEU zeolite at room temperature under solvent-free conditions. Bull Korean Chem Soc 33:2251–2253
Tajbakhsh M, Alinezhad H, Nasrollahzadeh M, Kamali TA (2016) Preparation, characterization and application of nanosized CuO/HZSM-5 as an efficient and heterogeneous catalyst for the N-formylation of amines at room temperature. J Coll Interface Sci 471:37–47
Ansari MI, Hussain MK, Yadav N, Gupta PK, Hajela K (2012) Silica supported perchloric acid catalyzed rapid N-formylation under solvent-free conditions. Tetrahedron Lett 53:2063–2065
Habibi D, Rahmani P, Akbaripanah Z (2013) N-Formylation of anilines with silica sulfuric acid under solvent-free conditions. J Chem. https://doi.org/10.1155/2013/972960
Serre C, Millange F, Thouvenot C, Nogues M, Marsolier G, Louerand D, Fґerey G (2002) Very large breathing effect in the first nanoporous chromium(III)-based solids: MIL-53 or CrIII(OH)·{O2C-C6H4-CO2}·{HO2C-C6H4-CO2H}x·H2Oy. J Am Chem Soc 124:13519–13526
Acknowledgments
This work was supported by the Ministry of Science and Higher Education of the Russian Federation within the governmental order for Boreskov Institute of Catalysis (AAAA-A21-121011390055-8).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Timofeeva, M.N., Panchenko, V.N., Lukoyanov, I.A. et al. Zirconium-containing metal organic frameworks as solid acid catalysts for the N-formylation of aniline with formic acid. Reac Kinet Mech Cat 133, 355–369 (2021). https://doi.org/10.1007/s11144-021-01982-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-01982-1