Abstract
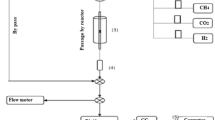
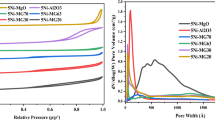
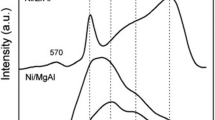
The nickel-based catalysts supported on MgO-modified α-Al2O3, CeO2, and SBA-15 were prepared by impregnation method and investigated by N2 physisorption measurements, powder X-ray diffraction, Raman spectroscopy, H2 temperature-programmed reduction, CO2 temperature-programmed desorption, and transmission electron microscopy. Investigation of the kinetics of the dry reforming of methane (DRM) was carried out in gradientless circulating micro-flow system at atmospheric pressure and temperature range of 600–800 °C. The results showed that carriers have a prominent role in characterising the physico-chemical properties of catalysts such as specific surface area, dispersity of active metal, reducibility and basicity that greatly affect the adsorption feature and activity of NiO catalyst. However, the kinetic equation of DRM on three catalysts was found to be written by a common fractional equation, following a dual-site Langmuir–Hinshelwood Hougen Watson model. The order in the catalyst reducibility and apparent rate constant was observed as follows: NiMg/Al<Ni/Ce<Ni/SBA, while the apparent activation energy (E) is in the opposite order. The highest activity was observed on the catalyst containing 31.2 wt% Ni supported on SBA-15.






Similar content being viewed by others
References
Guo J, Lou H, Zhao H, Chai D, Zheng X (2004) Dry reforming of methane over nickel catalysts supported on magnesium aluminate spinels. Appl Catal A 273(1–2):75–82
Roh H-S, Jun K-W (2008) Carbon dioxide reforming of methane over Ni catalysts supported on Al2O3 modified with La2O3, MgO, and CaO. Catal Surv Asia 12(4):239–252
Nakamura J, Aikawa K, Sato K, Uchijima T (1994) Role of support in reforming of CH4 with CO2 over Rh catalysts. Catal Lett 25(3–4):265–270
Koo KY, Roh H-S, Seo YT, Seo DJ, Yoon WL, Park SB (2008) A highly effective and stable nano-sized Ni/MgO–Al2O3 catalyst for gas to liquids (GTL) process. Int J Hydrogen Energy 33(8):2036–2043
Mazumder J, de Lasa H (2014) Fluidizable Ni/La2O3–γAl2O3 catalyst for steam gasification of a cellulosic biomass surrogate. Appl Catal B 160:67–79
Wang Y, Fang Q, Shen W, Zhu Z, Fang Y (2018) (Ni/MgAl2O4)@ SiO2 core–shell catalyst with high coke-resistance for the dry reforming of methane. React Kinet Mech Catal 125(1):127–139
Silva CK, Baston EP, Melgar LZ, Bellido JD (2019) Ni/Al2O3–La2O3 catalysts synthesized by a one-step polymerization method applied to the dry reforming of methane: effect of precursor structures of nickel, perovskite and spinel. React Kinet Mech Catal 128(1):251–269
Xu Z, Li Y, Zhang J, Chang L, Zhou R, Duan Z (2001) Ultrafine NiO–La2O3–Al2O3 aerogel: a promising catalyst for CH4/CO2 reforming. Appl Catal A 213(1):65–71
Nematollahi B, Rezaei M, Khajenoori M (2011) Combined dry reforming and partial oxidation of methane to synthesis gas on noble metal catalysts. Int J Hydrogen Energy 36(4):2969–2978
Charisiou ND, Siakavelas G, Papageridis KN, Baklavaridis A, Tzounis L, Avraam DG, Goula MA (2016) Syngas production via the biogas dry reforming reaction over nickel supported on modified with CeO2 and/or La2O3 alumina catalysts. J Nat Gas Sci Eng 31:164–183. https://doi.org/10.1016/j.jngse.2016.02.021
Fornasiero P, Dimonte R, Rao GR, Kaspar J, Meriani S, Trovarelli A, Graziani M (1995) Rh-loaded CeO2–ZrO2 solid-solutions as highly efficient oxygen exchangers: dependence of the reduction behavior and the oxygen storage capacity on the structural-properties. J Catal 151(1):168–177. https://doi.org/10.1006/jcat.1995.1019
Zhang J, Kumagai H, Yamamura K, Ohara S, Takami S, Morikawa A, Shinjoh H, Kaneko K, Adschiri T, Suda A (2011) Extra-low-temperature oxygen storage capacity of CeO2 nanocrystals with cubic facets. Nano Lett 11(2):361–364
Wu L, Wiesmann HJ, Moodenbaugh AR, Klie RF, Zhu Y, Welch DO, Suenaga M (2004) Oxidation state and lattice expansion of CeO2−x nanoparticles as a function of particle size. Phys Rev B 69(12):125415. https://doi.org/10.1103/PhysRevB.69.125415
Tomishige K, Asadullah M, Kunimori K (2003) Novel catalysts for gasification of biomass with high conversion efficiency. Catal Surv Asia 7(4):219–233
Nunan JG, Robota HJ, Cohn MJ, Bradley SA (1992) Physicochemical properties of Ce-containing three-way catalysts and the effect of Ce on catalyst activity. J Catal 133(2):309–324
Akri M, Zhao S, Li X, Zang K, Lee AF, Isaacs MA, Xi W, Gangarajula Y, Luo J, Ren Y (2019) Atomically dispersed nickel as coke-resistant active sites for methane dry reforming. Nat Commun 10(1):1–10
Ramli A, Mohamad MF, Yusup S, Hin TYY (2016) Hydrogen production from gasification of palm kernel shell in the presence of Fe/CeO2 catalysts. Malays J Anal Sci 20(2):303–308
Omoregbe O, Danh HT, Nguyen-Huy C, Setiabudi H, Abidin S, Truong QD, Vo D-VN (2017) Syngas production from methane dry reforming over Ni/SBA-15 catalyst: effect of operating parameters. Int J Hydrogen Energy 42(16):11283–11294
Zhang Q, Wang M, Zhang T, Wang Y, Tang X, Ning P (2015) A stable Ni/SBA-15 catalyst prepared by the ammonia evaporation method for dry reforming of methane. RSC Adv 5(114):94016–94024
Harley-Trochimczyk A, Pham T, Chang J, Chen E, Worsley MA, Zettl A, Mickelson W, Maboudian R (2016) Platinum nanoparticle loading of boron nitride aerogel and its use as a novel material for low-power catalytic gas sensing. Adv Func Mater 26(3):433–439
Bu K, Deng J, Zhang X, Kuboon S, Yan T, Li H, Shi L, Zhang D (2020) Promotional effects of B-terminated defective edges of Ni/boron nitride catalysts for coking-and sintering-resistant dry reforming of methane. Appl Catal B 267:118692
Cao Y, Maitarad P, Gao M, Taketsugu T, Li H, Yan T, Shi L, Zhang D (2018) Defect-induced efficient dry reforming of methane over two-dimensional Ni/h-boron nitride nanosheet catalysts. Appl Catal B 238:51–60
Cao Y, Lu M, Fang J, Shi L, Zhang D (2017) Hexagonal boron nitride supported mesoSiO2-confined Ni catalysts for dry reforming of methane. Chem Commun 53(54):7549–7552
Lu M, Zhang X, Deng J, Kuboon S, Faungnawakij K, Xiao S, Zhang D (2020) Coking-resistant dry reforming of methane over BN-nanoceria interface-confined Ni catalysts. Catal Sci Technol 10:4237–4244
Bu K, Kuboon S, Deng J, Li H, Yan T, Chen G, Shi L, Zhang D (2019) Methane dry reforming over boron nitride interface-confined and LDHs-derived Ni catalysts. Appl Catal B 252:86–97
Al-Fatesh AS, Fakeeha AH, Abasaeed AE (2011) Effects of promoters on methane dry reforming over Ni catalyst on a mixed (a-Al2O3+TiO2-P25) support. Int J Phys Sci 6(36):8083–8092
Bradford MC, Vannice MA (1999) CO2 reforming of CH4 over supported Ru catalysts. J Catal 183(1):69–75
Iyer MV, Norcio LP, Kugler EL, Dadyburjor DB (2003) Kinetic modeling for methane reforming with carbon dioxide over a mixed-metal carbide catalyst. Ind Eng Chem Res 42(12):2712–2721
Wei J, Iglesia E (2004) Structural requirements and reaction pathways in methane activation and chemical conversion catalyzed by rhodium. J Catal 225(1):116–127
Solh TE, Jarosch K, de Lasa H (2003) Catalytic dry reforming of methane in a CREC riser simulator kinetic modeling and model discrimination. Ind Eng Chem Res 42(12):2507–2515
Gokon N, Yamawaki Y, Nakazawa D, Kodama T (2011) Kinetics of methane reforming over Ru/γ-Al2O3-catalyzed metallic foam at 650–900 °C for solar receiver-absorbers. Int J Hydrogen Energy 36(1):203–215
Daza CE, Kiennemann A, Moreno S, Molina R (2009) Dry reforming of methane using Ni–Ce catalysts supported on a modified mineral clay. Appl Catal A 364(1–2):65–74
Akpan E, Sun Y, Kumar P, Ibrahim H, Aboudheir A, Idem R (2007) Kinetics, experimental and reactor modeling studies of the carbon dioxide reforming of methane (CDRM) over a new Ni/CeO2–ZrO2 catalyst in a packed bed tubular reactor. Chem Eng Sci 62(15):4012–4024
Gokon N, Osawa Y, Nakazawa D, Hatamachi T, Kodama T (2008) Kinetics of CO2 reforming of methane by catalytically activated metallic foam absorber for solar receiver-reactors. ASME 2008 2nd International Conference on Energy Sustainability collocated with the Heat Transfer, Fluids Engineering, and 3rd Energy Nanotechnology Conferences. American Society of Mechanical Engineers, Florida, pp 371–383
Wei J, Iglesia E (2004) Isotopic and kinetic assessment of the mechanism of reactions of CH4 with CO2 or H2O to form synthesis gas and carbon on nickel catalysts. J Catal 224(2):370–383
Bhat R, Sachtler W (1997) Potential of zeolite supported rhodium catalysts for the CO2 reforming of CH4. Appl Catal A 150(2):279–296
Mark MF, Maier WF (1994) Active surface carbon—a reactive Intermediate in the production of synthesis gas from methane and carbon dioxide. Angew Chem, Int Ed Engl 33(15–16):1657–1660
Rostrupnielsen J, Hansen JB (1993) CO2-reforming of methane over transition metals. J Catal 144(1):38–49
Zheng X, Tan S, Dong L, Li S, Chen H (2015) Silica-coated LaNiO3 nanoparticles for non-thermal plasma assisted dry reforming of methane: experimental and kinetic studies. Chem Eng J 265:147–156
Erdohelyi A, Cserényi J, Solymosi F (1993) Activation of CH4 and its reaction with CO2 over supported Rh catalysts. J Catal 141(1):287–299
Mark MF, Maier WF, Mark F (1997) Reaction kinetics of the CO2 reforming of methane. Chem Eng Technol Ind Chem Plant Equip Process Eng Biotechnol 20(6):361–370
Li Y, Wang Y, Zhang X, Mi Z (2008) Thermodynamic analysis of autothermal steam and CO2 reforming of methane. Int J Hydrogen Energy 33(10):2507–2514
Wei J, Iglesia E (2004) Mechanism and site requirements for activation and chemical conversion of methane on supported Pt clusters and turnover rate comparisons among noble metals. J Phys Chem B 108(13):4094–4103
Wei J, Iglesia E (2004) Reaction pathways and site requirements for the activation and chemical conversion of methane on Ru-based catalysts. J Phys Chem B 108(22):7253–7262
Verykios XE (2003) Catalytic dry reforming of natural gas for the production of chemicals and hydrogen. Int J Hydrogen Energy 28(10):1045–1063
Ginsburg JM, Piña J, El Solh T, De Lasa HI (2005) Coke formation over a nickel catalyst under methane dry reforming conditions: thermodynamic and kinetic models. Ind Eng Chem Res 44(14):4846–4854
Foo SY, Cheng CK, Nguyen T-H, Adesina AA (2011) Kinetic study of methane CO2 reforming on Co–Ni/Al2O3 and Ce–Co–Ni/Al2O3 catalysts. Catal Today 164(1):221–226
Ayodele BV, Khan MR, Lam SS, Cheng CK (2016) Production of CO-rich hydrogen from methane dry reforming over lanthania-supported cobalt catalyst: kinetic and mechanistic studies. Int J Hydrogen Energy 41(8):4603–4615
Benguerba Y, Virginie M, Dumas C, Ernst B (2017) Methane dry reforming over Ni–Co/Al2O3: kinetic modelling in a catalytic fixed-bed reactor. Int J Chem Reactor Eng 15(6):20160170
Bradford MC, Vannice MA (1996) Catalytic reforming of methane with carbon dioxide over nickel catalysts II. Reaction kinetics. Appl Catal A 142(1):97–122
Tsipouriari VA, Verykios XE (2001) Kinetic study of the catalytic reforming of methane with carbon dioxide to synthesis gas over Ni/La2O3 catalyst. Catal Today 64(1–2):83–90
Olsbye U, Wurzel T, Mleczko L (1997) Kinetic and reaction engineering studies of dry reforming of methane over a Ni/La/Al2O3 catalyst. Ind Eng Chem Res 36(12):5180–5188
Pichas C, Pomonis P, Petrakis D, Ladavos A (2010) Kinetic study of the catalytic dry reforming of CH4 with CO2 over La2−xSrxNiO4 perovskite-type oxides. Appl Catal A 386(1–2):116–123
Cheng CK, Foo SY, Adesina AA (2010) Glycerol steam reforming over bimetallic Co-Ni/Al2O3. Ind Eng Chem Res 49(21):10804–10817
Barroso Quiroga MM, Castro Luna AE (2007) Kinetic analysis of rate data for dry reforming of methane. Ind Eng Chem Res 46(16):5265–5270
Luo J, Yu Z, Ng C, Au C (2000) CO2/CH4 reforming over Ni–La2O3/5A: an investigation on carbon deposition and reaction steps. J Catal 194(2):198–210
Foppa L, Margossian T, Kim SM, Müller C, Copéret C, Larmier K, Comas-Vives A (2017) Contrasting the role of Ni/Al2O3 interfaces in water–gas shift and dry reforming of methane. J Am Chem Soc 139(47):17128–17139
Liu Z, Grinter DC, Lustemberg PG, Nguyen-Phan TD, Zhou Y, Luo S, Waluyo I, Crumlin EJ, Stacchiola DJ, Zhou J (2016) Dry reforming of methane on a highly-active Ni–CeO2 catalyst: Effects of metal-support interactions on C−H bond breaking. Angew Chem Int Ed 55(26):7455–7459
Burghgraef H, Jansen A, Van Santen R (1995) Methane activation and dehydrogenation on nickel and cobalt: a computational study. Surf Sci 324(2–3):345–356
Kroll V, Swaan H, Lacombe S, Mirodatos C (1996) Methane reforming reaction with carbon dioxide over Ni/SiO2 catalyst: II. A mechanistic study. J Catal 164(2):387–398
Chang J-S, Park S-E, Yoo JW, Park J-N (2000) Catalytic behavior of supported KNiCa catalyst and mechanistic consideration for carbon dioxide reforming of methane. J Catal 195(1):1–11
Tang S, Ji L, Lin J, Zeng H, Tan K, Li K (2000) CO2 reforming of methane to synthesis gas over sol–gel-made Ni/γ–Al2O3 catalysts from organometallic precursors. J Catal 194(2):424–430
Gamman JJ, Millar JG, Rose G, Drennan J (1998) Characterisation of SiO2-supported nickel catalysts for carbon dioxide reforming of methane. J Chem Soc Faraday Trans 94(5):701–710. https://doi.org/10.1039/A706730E
Osaki T, Horiuchi T, Suzuki K, Mori T (1997) CH4/CD4 isotope effect on the reaction of adsorbed hydrocarbon species in CO2-reforming over Ni/Al2O3 catalyst. Catal Lett 44(1–2):19–21
Osaki T, Mori T (2001) Role of potassium in carbon-free CO2 reforming of methane on K-promoted Ni/Al2O3 catalysts. J Catal 204(1):89–97
Schuurman Y, Marquez-Alvarez C, Kroll VCH, Mirodatos C (1998) Unraveling mechanistic features for the methane reforming by carbon dioxide over different metals and supports by TAP experiments. Catal Today 46:185–192
Nandini A, Pant K, Dhingra S (2006) Kinetic study of the catalytic carbon dioxide reforming of methane to synthesis gas over Ni–K/CeO2–Al2O3 catalyst. Appl Catal A 308:119–127
Azarhoosh MJ, Halladj R, Askari S (2017) Presenting a new kinetic model for methanol to light olefins reactions over a hierarchical SAPO-34 catalyst using the Langmuir–Hinshelwood–Hougen–Watson mechanism. J Phys: Condens Matter 29(42):425202
Rahimi N, Karimzadeh R (2015) Kinetic modeling of catalytic cracking of C4 alkanes over La/HZSM-5 catalysts in light olefin production. J Anal Appl Pyrol 115:242–254
Sawatmongkhon B, Theinnoi K, Wongchang T, Haoharn C, Tsolakis A (2017) Combination of Langmuir-Hinshelwood-Hougen-Watson and microkinetic approaches for simulation of biogas dry reforming over a platinum-rhodium alumina catalyst. Int J Hydrogen Energy 42(39):24697–24712
Arsalanfar M, Mirzaei A, Atashi H, Bozorgzadeh H, Vahid S, Zare A (2012) An investigation of the kinetics and mechanism of Fischer-Tropsch synthesis on Fe–Co–Mn supported catalyst. Fuel Process Technol 96:150–159
Vahid S, Mirzaei A (2014) An investigation of the kinetics and mechanism of Fischer-Tropsch synthesis on Fe–Co–Ni supported catalyst. J Ind Eng Chem 20(4):2166–2173
Loc LC, Phuong PH, Putthea D, Tri N, Van NTT, Cuong HT (2018) Effect of CeO2 morphology on performance of NiO/CeO2 catalyst in combined steam and CO2 reforming of CH4. Int J Nanotechnol 15(11–12):968–982
Phuong PH, Loc LC, Cuong HT, Tri N (2018) Effect of NiO loading and thermal treatment duration on performance of Ni/SBA-15 catalyst in combined steam and CO2 reforming of CH4. Mater Trans 59(12):1898–1902
Loc LC, Phuong PH, Thao NHP, Tri N, Van NTT, Cuong HT, Anh HC (2017) Influence of preparation method on the activity of NiO+MgO/Al2O3 catalyst in dry reforming of methane. Vietnam J Chem 55(3E):1–7
Kiperman SL (1978) Kinetic Models in Heterogeneous Catalysis. Russ Chem Rev 47(1):1
Temkin MI (1976) Relaxation speed of two-stage catalytic reaction. Kinet Catal 17(5):1095–1099
Zhao D, Feng J, Huo Q, Melosh N, Fredrickson GH, Chmelka BF, Stucky GD (1998) Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 279(5350):548–552
Bukhari S, Chin C, Setiabudi H, Vo D-VN (2017) Tailoring the properties and catalytic activities of Ni/SBA-15 via different TEOS/P123 mass ratios for CO2 reforming of CH4. J Environ Chem Eng 5(4):3122–3128
Rodriguez-Gomez A, Pereñiguez R, Caballero A (2018) Nickel particles selectively confined in the mesoporous channels of SBA-15 yielding a very stable catalyst for DRM reaction. J Phys Chem B 122(2):500–510
Aghamohammadi S, Haghighi M, Karimipour S (2013) A comparative synthesis and physicochemical characterizations of Ni/Al2O3–MgO nanocatalyst via sequential impregnation and sol–gel methods used for CO2 reforming of methane. J Nanosci Nanotechnol 13(7):4872–4882
Sun Y, Jiang E, Xu X, Wang J, Li Z (2018) Supplied oxygen properties of NiO/NiAl2O4 in chemical looping re-forming of biomass pyrolysis gas: the influence of synthesis method. ACS Sustain Chem Eng 6(11):14660–14668
Liu D, Quek XY, Cheo WNE, Lau R, Borgna A, Yang Y (2009) MCM-41 supported nickel-based bimetallic catalysts with superior stability during carbon dioxide reforming of methane: effect of strong metal–support interaction. J Catal 266(2):380–390
Walker DM, Pettit SL, Wolan JT, Kuhn JN (2012) Synthesis gas production to desired hydrogen to carbon monoxide ratios by tri-reforming of methane using Ni–MgO–(Ce, Zr)O2 catalysts. Appl Catal A 445:61–68
Ay H, Üner D (2015) Dry reforming of methane over CeO2 supported Ni, Co and Ni–Co catalysts. Appl Catal B 179:128–138
Li B, Zhang S (2013) Methane reforming with CO2 using nickel catalysts supported on yttria-doped SBA-15 mesoporous materials via sol–gel process. Int J Hydrogen Energy 38(33):14250–14260
Liu H, Wang H, Shen J, Sun Y, Liu Z (2008) Promotion effect of cerium and lanthanum oxides on Ni/SBA-15 catalyst for ammonia decomposition. Catal Today 131(1–4):444–449
Luo C, Li D, Wu W, Zhang Y, Pan C (2014) Preparation of porous micro–nano-structure NiO/ZnO heterojunction and its photocatalytic property. RSC Adv 4(6):3090–3095
Agarwal S, Zhu X, Hensen E, Mojet B, Lefferts L (2015) Surface-dependence of defect chemistry of nanostructured ceria. J Phys Chem C 119(22):12423–12433
Gamarra D, Munuera G, Hungría A, Fernández-García M, Conesa J, Midgley P, Wang X, Hanson J, Rodríguez J, Martínez-Arias A (2007) Structure−activity relationship in nanostructured copper−ceria-based preferential CO oxidation catalysts. J Phys Chem C 111(29):11026–11038
Sudarsanam P, Hillary B, Mallesham B, Rao BG, Amin MH, Nafady A, Alsalme AM, Reddy BM, Bhargava SK (2016) Designing CuOx nanoparticle-decorated CeO2 nanocubes for catalytic soot oxidation: role of the nanointerface in the catalytic performance of heterostructured nanomaterials. Langmuir 32(9):2208–2215
Zheng X, Li Y, Zhang L, Shen L, Xiao Y, Zhang Y, Au C, Jiang L (2019) Insight into the effect of morphology on catalytic performance of porous CeO2 nanocrystals for H2S selective oxidation. Appl Catal B 252:98–110
Li Y, Wei Z, Gao F, Kovarik L, Baylon RA, Peden CH, Wang Y (2015) Effect of oxygen defects on the catalytic performance of VOx/CeO2 catalysts for oxidative dehydrogenation of methanol. ACS Catal 5(5):3006–3012
Ocsachoque M, Pompeo F, Gonzalez G (2011) Rh–Ni/CeO2–Al2O3 catalysts for methane dry reforming. Catal Today 172(1):226–231
Djaidja A, Libs S, Kiennemann A, Barama A (2006) Characterization and activity in dry reforming of methane on NiMg/Al and Ni/MgO catalysts. Catal Today 113(3–4):194–200
Schulze K, Makowski W, Chyży R, Dziembaj R, Geismar G (2001) Nickel doped hydrotalcites as catalyst precursors for the partial oxidation of light paraffins. Appl Clay Sci 18(1–2):59–69
Roh H-S, Jun K-W, Dong W-S, Chang J-S, Park S-E, Joe Y-I (2002) Highly active and stable Ni/Ce–ZrO2 catalyst for H2 production from methane. J Mol Catal A: Chem 181(1–2):137–142
Li Y, Xie X, Liu J, Cai M, Rogers J, Shen W (2008) Synthesis of α-Ni(OH)2 with hydrotalcite-like structure: precursor for the formation of NiO and Ni nanomaterials with fibrous shapes. Chem Eng J 136(2–3):398–408
Du X, Zhang D, Shi L, Gao R, Zhang J (2012) Morphology dependence of catalytic properties of Ni/CeO2 nanostructures for carbon dioxide reforming of methane. J Phys Chem C 116(18):10009–10016
Jacobs G, Das TK, Zhang Y, Li J, Racoillet G, Davis BH (2002) Fischer-Tropsch synthesis: support, loading, and promoter effects on the reducibility of cobalt catalysts. Appl Catal A 233(1–2):263–281
He L, Liang B, Li L, Yang X, Huang Y, Wang A, Wang X, Zhang T (2015) Cerium-oxide-modified nickel as a non-noble metal catalyst for selective decomposition of hydrous hydrazine to hydrogen. ACS Catal 5(3):1623–1628
Sidik S, Triwahyono S, Jalil A, Majid Z, Salamun N, Talib N, Abdullah T (2016) CO2 reforming of CH4 over Ni–Co/MSN for syngas production: role of Co as a binder and optimization using RSM. Chem Eng J 295:1–10
Das S, Ashok J, Bian Z, Dewangan N, Wai M, Du Y, Borgna A, Hidajat K, Kawi S (2018) Silica-Ceria sandwiched Ni core–shell catalyst for low temperature dry reforming of biogas: coke resistance and mechanistic insights. Appl Catal B 230:220–236
Bian Z, Li Z, Ashok J, Kawi S (2015) A highly active and stable Ni–Mg phyllosilicate nanotubular catalyst for ultrahigh temperature water-gas shift reaction. Chem Commun 51(91):16324–16326
Xu D, Li W, Ge Q, Xu H (2005) A novel process for converting coalmine-drained methane gas to syngas over nickel–magnesia solid solution catalysts. Fuel Process Technol 86(9):995–1006
Yoshida T, Tanaka T, Yoshida H, Funabiki T, Yoshida S (1996) Study on the dispersion of nickel ions in the NiO− MgO system by X-ray absorption fine structure. J Phys Chem 100(6):2302–2309
Zeng Y, Ma H, Zhang H, Ying W, Fang D (2014) Highly efficient NiAl2O4-free Ni/γ-Al2O3 catalysts prepared by solution combustion method for CO methanation. Fuel 137:155–163
Wang X, Zhu L, Liu Y, Wang S (2018) CO2 methanation on the catalyst of Ni/MCM-41 promoted with CeO2. Sci Total Environ 625:686–695
Xu B, Zhang Q, Yuan S, Zhang M, Ohno T (2015) Morphology control and characterization of broom-like porous CeO2. Chem Eng J 260:126–132
Chong CC, Teh LP, Setiabudi HD (2019) Syngas production via CO2 reforming of CH4 over Ni-based SBA-15: promotional effect of promoters (Ce, Mg, and Zr). Mater Today Energy 12:408–417
Dholabhai PP, Adams JB, Crozier P, Sharma R (2010) Oxygen vacancy migration in ceria and Pr-doped ceria: A DFT+U study. J Chem Phys 132(9):094104
Binet C, Badri A, Boutonnet-Kizling M, Lavalley J-C (1994) FTIR study of carbon monoxide adsorption on ceria: CO2–2 carbonite dianion adsorbed species. J Chem Soc Faraday Trans 90(7):1023–1028
Binet C, Daturi M, Lavalley J-C (1999) IR study of polycrystalline ceria properties in oxidised and reduced states. Catal Today 50(2):207–225
Song Z, Liu W, Nishiguchi H (2007) Quantitative analyses of oxygen release/storage and CO2 adsorption on ceria and Pt–Rh/ceria. Catal Commun 8(4):725–730
Hojo H, Mizoguchi T, Ohta H, Findlay SD, Shibata N, Yamamoto T, Ikuhara Y (2010) Atomic structure of a CeO2 grain boundary: the role of oxygen vacancies. Nano Lett 10(11):4668–4672
Ganduglia-Pirovano MV, Da Silva JL, Sauer J (2009) Density-functional calculations of the structure of near-surface oxygen vacancies and electron localization on CeO2 (111). Phys Rev Lett 102(2):026101
Naeem MA, Al-Fatesh AS, Abasaeed AE, Fakeeha AH (2014) Activities of Ni-based nano catalysts for CO2–CH4 reforming prepared by polyol process. Fuel Process Technol 122:141–152
Alipour Z, Rezaei M, Meshkani F (2014) Effect of Ni loadings on the activity and coke formation of MgO-modified Ni/Al2O3 nanocatalyst in dry reforming of methane. J Energy Chem 23(5):633–638
Wang S, Lu G, Millar GJ (1996) Carbon dioxide reforming of methane to produce synthesis gas over metal-supported catalysts: state of the art. Energy Fuels 10(4):896–904
Das S, Sengupta M, Patel J, Bordoloi A (2017) A study of the synergy between support surface properties and catalyst deactivation for CO2 reforming over supported Ni nanoparticles. Appl Catal A 545:113–126
Li Z, Das S, Hongmanorom P, Dewangan N, Wai MH, Kawi S (2018) Silica-based micro-and mesoporous catalysts for dry reforming of methane. Catal Sci Technol 8(11):2763–2778
Zambrano D, Soler J, Herguido J, Menéndez M (2019) Kinetic study of dry reforming of methane over Ni–Ce/Al2O3 catalyst with deactivation. Top Catal 62:456–466
Cj L, Ye J, Jiang J, Pan Y (2011) Progresses in the preparation of coke resistant Ni-based catalyst for steam and CO2 reforming of methane. ChemCatChem 3(3):529–541
Danilova M, Fedorova Z, Kuzmin V, Zaikovskii V, Porsin A, Krieger T (2015) Combined steam and carbon dioxide reforming of methane over porous nickel based catalysts. Catal Sci Technol 5(5):2761–2768
Li KZ, Wang H, Wei YG, Yan DX (2009) Selective oxidation of carbon using iron-modified cerium oxide. J Phys Chem C 113(34):15288–15297
Lercher J, Bitter J, Hally W, Niessen W, Seshan K (1996) Design of stable catalysts for methane-carbon dioxide reforming. Stud Surf Sci Catal 101:463–472
Bradford MC, Vannice MA (1999) The role of metal–support interactions in CO2 reforming of CH4. Catal Today 50(1):87–96
Kapil A, Wilson K, Lee AF, Sadhukhan J (2011) Kinetic modeling studies of heterogeneously catalyzed biodiesel synthesis reactions. Ind Eng Chem Res 50(9):4818–4830
Ayodele BV, Hossain SS, Lam SS, Osazuwa OU, Khan MR, Cheng CK (2016) Syngas production from CO2 reforming of methane over neodymium sesquioxide supported cobalt catalyst. J Nat Gas Sci Eng 34:873–885
Acknowledgements
This work was supported by the program code VAST03.01/2019-2020 from the Materials Science Council, Vietnam Academy of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nguyen, T., Luu, C.L., Phan, H.P. et al. Methane dry reforming over nickel-based catalysts: insight into the support effect and reaction kinetics. Reac Kinet Mech Cat 131, 707–735 (2020). https://doi.org/10.1007/s11144-020-01876-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-020-01876-8