Abstract
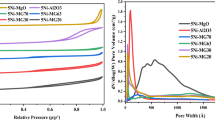
In order to investigate the surface structure changes of nickel-based catalysts during the syngas methanation process, nickel-based catalysts were prepared with impregnation method and the tests were carried out in a fluidized bed reactor. The test results showed that the catalytic performance declined rapidly with the reaction time. Energy dispersive spectroscopy measurements of the reacted catalyst were carried out and the results indicated that there was carbon observed on the catalysts surface. The carbon deposited on the catalyst surface was removed in a thermogravimetric analyzer to obtain the regenerated catalyst and it was found that the amount of the accumulated carbon on the catalyst surface was 2.09 wt% of the reacted catalyst. N2 adsorption–desorption measurements were conducted on the fresh catalyst, reacted catalyst and regenerated catalyst. The specific area of the reacted catalyst decreased significantly and could not restore to the original level even after removing the deposited carbon. The pore volume of reacted catalyst increased 16.67% compared to the fresh catalyst while the average pore diameter remained almost no change. The pores existing on the fresh catalyst were mainly the mesopores while the pore size distribution expanded and the macrospores came into being on the reacted catalyst. It can be concluded that a kind of stable carbon was produced on the catalyst surface after methanation process, which caused dramatic changes to the surface textural properties.






Similar content being viewed by others
References
Carbo MC, Smit R, van der Drift B, Jansen D (2011) Bio Energy with CCS (BECCS): Large potential for BioSNG at low CO2 avoidance cost. Energy Procedia 4:2950–2954
Martin G, François M (2009) Thermo-economic process model for thermochemical production of synthetic natural gas (SNG) from lignocellulosic biomass. Biomass Bioenergy 33:1587–1604
Ahrenfeldt J, Jørgensen B, Thomsen T (2010) Bio-SNG potential assessment: Denmark 2020. Danmarks Tekniske Universitet, Risø Nationallaboratoriet for Bæredygtig Energi
Kopyscinski J, Schildhauer TJ, Biollaz SM (2010) Production of synthetic natural gas (SNG) from coal and dry biomass–A technology review from 1950 to 2009. Fuel 89(8):1763–1783
Wu HX, Zhao ZL, Wang XB, Zheng AQ, Li HB, He F (2013) Technical development on synthetic natural gas production from biomass (Bio-SNG). Chem Ind Eng Prog 32(83–90):113 (in Chinese with English abstract)
Wang B, Yu W, Meng D, Li Z, Xu Y, Ma X (2018) Effect of citric acid on CoO-MoO3/Al2O3 catalysts for sulfur-resistant methanation. Reac Kinet Mech Cat 125:111–126
Ping D, Zhao H, Dong X (2018) Ni-doped TiO2 nanotubes supported Ru catalysts for CO selective methanation in H2-rich reformate gases. Reac Kinet Mech Cat 124:619–631
He HP, Hill JM (2007) Carbon deposition on Ni/YSZ composites exposed to humidified methane. Appl Catal A 317(2):284–292
Bai X, Wang S, Sun T, Wang S (2014) Influence of operating conditions on carbon deposition over a Ni catalyst for the production of synthetic natural gas (SNG) from coal. Catal Lett 144(12):2157–2166
Liu Q, Gu F, Gao J, Li H, Xu G, Su F (2014) Coking-resistant Ni-ZrO2/Al2O3 catalyst for CO methanation. J Energy Chem 23(6):761–770
Yang X, Gao G, Shi Q, Wang X, Zhang J, Han C, Tong M (2014) Impact of mesoporous structure of acid-treated clay on nickel dispersion and carbon deposition for CO methanation. Int J Hydrogen Energy 39(7):3231–3242
Barrientos J, Lualdi M, Suárez París R, Montes V, Boutonnet M (2015) CO methanation over TiO2-supported nickel catalysts: a carbon formation study. Appl Catal A 502:276–286
Feng F, Song GH, Zhang DH, Feng X, Jin L, Zhang L (2018) Experiment and macro-kinetics on biomass syngas methanation in a fluidized bed reactor. Chem React Eng Technol 34(5):460–466 (in Chinese with English abstract)
Feng F, Song GH, Shen LH, Xiao J (2013) Design and operation of biomass methanation reactor via pressurized fluidized bed technology. Chemistry 76(9):828–832 (in Chinese with English abstract)
Moulijn JA, Diepen AEV, Kapteijn F (2001) Catalyst deactivation: is it predictable? What to do? Appl Catal A Gen 212(1):3–16
Shen WM, Dumesic JA, Hill CG (1981) Criteria for stable Ni particle size under methanation reaction conditions: nickel transport and particle size growth via nickel carbonyl. J Catal 68(1):152–165
Dagle RA, Wang Y, Xia GG, Strohm JJ, Holladay J, Palo DR (2007) Selective CO methanation catalysts for fuel processing applications. Appl Catal A 326(2):213–218
Zyryanova MM, Snytnikov PV, Amosov YI, Kuzmin VA, Kirillov VA, Sobyanin VA (2011) Design, scale-out, and operation of a preferential CO methanation reactor with a nickel–ceria catalyst. Chem Eng J 176:106–113
Liu B, Ji S (2013) Comparative study of fluidized-bed and fixed-bed reactor for syngas methanation over Ni-W/TiO2-SiO2 catalyst. J Energy Chem 22(5):740–746
Bartholomew CH (1982) Carbon deposition in steam reforming and methanation. Catal Rev 24(1):67–112
Mccarty JG, Wise H (1979) Hydrogenation of surface carbon on alumina-supported nickel. J Catal 57(3):406–416
Bartholomew CH, Weatherbee GD, Jarvi GA (1980) Effects of carbon deposits on the specific activity of nickel and nickel bimetallic catalysts. Chem Eng Commun 5(1–4):125–134
Rostrup-Nielsen J, Trimm DL (1977) Mechanisms of carbon formation on nickel-containing catalysts. J Catal 48(1–3):155–165
Erekson EJ, Sughrue EL, Bartholomew CH (1981) Catalyst degradation in high temperature methanation. Fuel Process Technol 5(1–2):91–101
Acknowledgements
This work was supported by Qing Lan Project of Jiangsu Province, China, the sponsorship of Jiangsu Oversea Research and Training Program for University Prominent Young and Middle-aged Teachers and Presidents.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Feng, F., Zhang, L., Huang, S. et al. Surface structure changes of nickel-based catalysts in the syngas methanation process. Reac Kinet Mech Cat 130, 229–240 (2020). https://doi.org/10.1007/s11144-020-01787-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-020-01787-8