Abstract
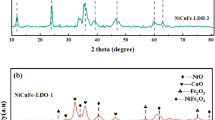
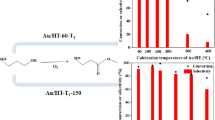
Three samples of NiAl layered double hydroxides with takovite-like structure were prepared by a co-precipitation method at Ni2+/Al3+ molar ratios of 1.5, 2.5 and 4.0. The samples were investigated as catalysts for hydrogen production by means of water–gas shift reaction (WGSR). The properties and catalytic behavior of the unmodified as-synthesized materials are compared with those of the same materials used as a support on which gold particles have been deposited. All samples were characterized by N2 physisorption, Powder X-ray diffraction (PXRD), and X-ray photoelectron spectroscopy (XPS) techniques. Catalytic activity of all materials was studied towards conversion of CO at atmospheric pressure within the temperature interval 140–300 °C. The dependence of WGS activity on the Ni2+/Al3+ molar ratio and the presence of gold were investigated. The promotional role of Au on the WGS performance was clearly demonstrated by Au–NiAl2.5 catalyst, which reached equilibrium conversion value of 97.6% at 240 °C. The stability test of the most active Au–NiAl2.5 catalyst resulted in the same CO conversion degree within 32 h under stream at 260 °C. A plausible scheme for the reaction mechanism, including the redox Ni2+ ↔ Ni3+ transition on the catalyst surface as well as adsorption and activation of the CO molecule on the Au particles, is proposed.




Similar content being viewed by others
References
Tao F, Ma Z (2013) Water-gas shift on gold catalysts: catalyst systems and fundamental studies. Phys Chem Chem Phys 15:15260–15270
Pal DB, Chand R, Upadhyay SN, Mishra PK (2018) Performance of water gas shift reaction catalysts: a review. Renew Sust Energy Rev 93:549–565
Reddy GK, Smirniotis PG (2015) Water gas shift reaction: research developments and applications. Elsevier, Amsterdam
Fuentes E, Jùnior A, Silva T, Assaf J, Rangel M (2011) A comparison between copper and nickel-based catalysts obtained from hydrotalcite-like precursors for WGSR. Catal Today 171:290–296
Andreev A, Idakiev V, Kostov K, Gabrovska M (1995) Water-gas shift reaction over nickel hydroxides. Catal Lett 31(2–3):245–252
Gabrovska M, Edreva-Kardjieva R, Idakiev V, Kunev B (2002) Influence of Mg on the Structure and the properties of Ni-Al takovite-like material. Bulg Chem Commun 34(3/4):395–404
Gabrovska M, Idakiev V, Tenchev K, Nikolova D, Edreva-Kardjieva R, Crisan D (2013) Catalytic performance of Ni-Al layered double hydroxides in CO purification processes. Russ J Phys Chem A 87(13):2152–2159
Reina TR, González MC, Palma S, Ivanova S, Odriozola JA (2014) Twenty years of golden future in the water gas shift reaction. In: Ma Z, Dai S (eds) Heterogeneous gold catalysts and catalysis, vol 18. RSC Catal Series, Royal Society of Chemistry, Cambridge, pp 111–140
Qi C, Amphlett JC, Peppley BA (2005) Methanol steam reforming over NiAl and Ni(M)Al layered double hydroxides (M = Au, Rh, Ir) derived catalysts. Cat Lett 104(1–2):57–62
Andreeva D, Idakiev V, Tabakova T, Andreev A (1996) Low-temperature water-gas shift reaction over Au/α-Fe2O3. J Catal 158:275–283
Wang X, Rodriguez JA, Hanson JC, Perez M, Evans J (2005) In situ time-resolved characterization of Au-CeO2 and AuOx-CeO2 catalysts during the water-gas shift reaction: presence of Au and O vacancies in the active phase. J Chem Phys 123:221101
Sandoval A, Gomez-Cortes A, Zanella R, Diaz G, Saniger JM (2007) Gold nanoparticles: support effects for the WGS reaction. J Mol Catal A 278:200–208
Odabasi C, Günay ME, Yildirim R (2014) Knowledge extraction for water gas shift reaction over noble metal catalysts from publications in the literature between 2002 and 2012. Int J Hydr Energy 39:5733–5746
Andreeva D, Tabakova T, Ilieva L (2013) Ceria-based gold catalysts: synthesis, properties and catalytic performance for the WGS and PROX processes. In: Trovarelli A, Fornasiero P (eds) Catalysis by ceria and related materials, 2nd edn. Imperial College Press, London, p 497
Flytzani-Stephanopoulos M (2014) Gold atoms stabilized on various supports catalyze the water-gas shift reaction. ACC Chem Res 47(3):783–792
Yang M, Flytzani-Stephanopoulos M (2017) Design of single-atom metal catalysts on various supports for the low temperature water-gas shift reaction. Catal Today 298:216–225
Bish D, Brindley G (1977) A reinvestigation of takovite, a nickel-aluminium hydroxycarbonate of the pyroaurite group. Am Miner 62(5–6):458–464
Cavani F, Trifirò F, Vaccari A (1991) Hydrotalcite-type anionic clays: preparation, properties and applications. Catal Today 11:173–301
Thevenot F, Szymanski R, Chaumette P (1989) Al-rich Zn-Al hydrotalcite-like compounds. Clays Clay Miner 37:396–402
Vaccari A (1998) Preparation and catalytic properties of cationic and anionic clays. Catal Today 41:53–71
Vaccari A (1999) Clays and catalysis: a promising future. Appl Clay Sci 14:161–198
Zümreoglu-Karan B, Ay A (2012) Layered double hydroxides—multifunctional nanomaterials. Chem Pap 66(1):1–10
Andreeva D, Idakiev V, Tabakova T, Ilieva L, Falaras P, Bourlinos A, Travlos A (2002) A low-temperature water-gas shift reaction over Au/CeO2 catalysts. Catal Today 72:51–57
Kozuch S, Martin JML (2012) “Turning Over” definitions in catalytic cycles. ACS Catal 2:2787–2794
Lente G (2013) Comment on “Turning Over” definitions in catalytic cycles. ACS Catal 3(3):381–382
Bligaard T, Morris RB, Campbell CT, Chen JG, Gates BC, Gorte RJ, Jones CW, Jones WD, Kitchin JR, Scott SL (2016) Toward benchmarking in catalysis science: best practices, challenges, and opportunities. ACS Catal 6:2590–2602
Guo D, Wang Y, Zhao P, Bai M, Xin H, Guo Z, Li J (2016) Selective aerobic oxidation of benzyl alcohol driven by visible light on gold nanoparticles supported on hydrotalcite modified by nickel ion. Catalysts 6(5):64
Benito P, Labajos F, Rives V (2006) Microwave-treated layered double hydroxides containing Ni2+ and Al3+: the effect of added Zn2+. J Solid State Chem 179:3784–3797
Gabrovska M, Edreva-Kardjieva R, Crişan D, Tzvetkov P, Shopska M, Shtereva I (2012) Ni-Al layered double hydroxides as catalyst precursors for CO2 removal by methanation. React Kinet Mech Cat 105(1):79–99
Kannan S, Narayanan A, Swamy CS (1996) Effect of composition on the physicochemical properties of nickel aluminium hydrotalcites. J Mater Sci 31:2353–2360
Shannon R (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chaleogenides. Acta Crystallogr A 32:751–767
Puxley DC, Kitchener IJ, Komodromos C, Parkyns ND (1983) The effect of preparation method upon the structures stability and metal/support interactions in nickel/alumina catalysts. Stud Surf Sci Catal 16:237–271
Rouquerol F, Rouquerol J, Sing K (1999) Adsorption by powders and porous solids, principle, methodology and applications. Academic Press, Cambridge
Thommes M, Kaneko K, Niemark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KSW (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87:1051–1069
Grosvenor AP, Biesinger MC, Smart RS, McIntyre NS (2006) New interpretations of XPS spectra of nickel metal and oxides. Surf Sci 600:1771–1779
Kim K, Winograd N (1974) X-ray photoelecton spectroscopic studies of nickel-oxygen surfaces using oxygen and argon ion-bombardment. Surf Sci 43:625–643
Barr TL (1978) An ESCA study of the termination of the passivation of elemental metals. J Phys Chem 82(16):1801–1810
Jindra J, Krejci I, Mrha J, Foekesson B, Johansson LY, Larsson R (1984) ESCA investigations on plastic-bonded nickel oxide electrodes. J Power Sources 13(2):123–136
Fang WH, Chen JS, Zhang QH, Deng WP, Wang Y (2011) Hydrotalcite-supported gold catalyst for the oxidant-free dehydrogenation of benzyl alcohol: studies on support and gold size effects. Chem Eur J 17:1247–1256
Oliva P, Leonardi J, Laurent J, Delmas C, Braconnier J, Figlarz M, Fievet F, de Guibert A (1982) Review of the structure and the electrochemistry of nickel hydroxides and oxy-hydroxides. J Power Sources 8:229–255
Tabakova T, Manzoli M, Vindigni F, Idakiev V, Boccuzzi F (2010) CO-free hydrogen production for fuel cell applications over Au/CeO2 Catalysts: FTIR insight into the role of dopant. J Phys Chem A 114:3909–3915
Boccuzzi F, Chiorino A, Manzoli M, Andreeva D, Tabakova T (1998) FTIR study of the low-temperature water-gas shift reaction on Au/Fe2O3 and Au/TiO2 catalysts. J Catal 188:176–185
Acknowledgements
The authors T.T. and I.I. gratefully acknowledge financial support by the Bulgarian National Science Fund (Contract ДH 09/5/2016). This work was partially supported by the Bulgarian Ministry of Education and Science under the National Research Programme E+: Low Carbon Energy for the Transport and Households, grant agreement D01-214/2018.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gabrovska, M., Tabakova, T., Ivanov, I. et al. Water–gas shift reaction over gold deposited on NiAl layered double hydroxides. Reac Kinet Mech Cat 127, 187–203 (2019). https://doi.org/10.1007/s11144-019-01572-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-019-01572-2