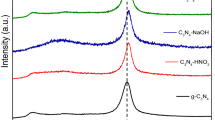
Abstract
We study the photolytic degradation of planar (PCB 2, PCB 12, PCB 13, PCB 15) and non-planar (PCB 8, PCB 29, PCB 31) polychlorobiphenyls in an aqueous alcohol under UV irradiation (λ = 240–320 nm) at ambient temperature and atmospheric pressure for 25 h on nanocrystalline TiO2 and CdS/TiO2. It is found that a conversion of PCB 2 (12.5%), PCB 12 (42.3%) and PCB 29 (98.0%) is more intense in the presence of the CdS/TiO2 composite, whereas mixture of the congeners PCB 8, PCB 13, PCB 15 and the congener PCB 31 can be photolyzed better in the presence of TiO2 with conversion 35.2% and 96.1%, respectively. The different conversions of the PCB congeners are explained considering the structures of the chloroaromatic radicals formed in situ as a result of the primary photolysis process and are confirmed by means of estimation of dipole moments calculated for singlet states of PCBs.




Similar content being viewed by others
References
Zanaveskin LN, Averyanov VA (1998) Polychlorobiphenyls: problems of the pollution of the environment and technological neutralisation methods. Russ Chem Rev 67:713–724
Gorbunova TI, Saloutin VI, Chupakhin ON (2010) Chemical methods of transformation of polychlorobiphenyls. Russ Chem Rev 79:511–530
Wu BZ, Chen HY, Wang SJ, Wai CM, Liao W, Chiu KH (2012) Reductive dechlorination for remediation of polychlorinated biphenyls. Chemosphere 88:757–768
Nadal M, Marquès M, Mari M, Domingo JL (2015) Climate change and environmental concentrations of POPs: a review. Environ Res 143:177–185
Teran T, Lamon L, Marcomini A (2012) Climate change effects on POPs’ environmental behaviour: a scientific perspective for future regulatory actions. Atmos Pollut Res 3:466–476
Sinkkonen S, Paasivirta J (2000) Degradation half-life times of PCDDs, PCDFs and PCBs for environmental fate modeling. Chemosphere 40:943–949
Bu Q, MacLeod M, Wong F, Toms LML, Mueller JF, Yu G (2015) Historical intake and elimination of polychlorinated biphenyls and organochlorine pesticides by the Australian population reconstructed from biomonitoring data. Environ Int 74:82–88
Wimmerová S, Lancz K, Tihányi J, Šovčiková E, Kočan A, Drobná B, Palkovičová L, Jurečková D, Fabišiková A, Čonka K, Trnovec T (2011) Half-lives of serum PCB congener concentrations in environmentally exposed early adolescents. Chemosphere 82:687–691
Hopf NB, Ruder AM, Waters MA, Succop P (2013) Concentration-dependent half-lives of polychlorinated biphenyl in sera from an occupational cohort. Chemosphere 91:172–178
Broding HC, Schettgen T, Gӧen T, Angerer J, Drexler H (2007) Development and verification of a toxicokinetic model of polychlorinated biphenyl elimination in persons working in a contaminated building. Chemosphere 68:1427–1434
Min JY, Kim R, Min KB (2014) Serum polychlorinated biphenyls concentrations and hearing impairment in adults. Chemosphere 102:6–11
Chen RC, Tang SY, Miyata H, Kashimoto T, Chang YC, Chang KJ, Tung TC (1985) Polychlorinated biphenyl poisoning: correlation of sensory and motor nerve conduction, neurologic symptoms, and blood levels of polychlorinated biphenyls, quaterphenyls, and dibenzofurans. Environ Res 37:340–348
Rempel AA, Kozlova EA, Gorbunova TI, Cherepanova SV, Gerasimov EY, Kozhevnikova NS, Valeeva AA, Korovin EY, Kaichev VV, Shchipunov YA (2015) Synthesis and solar light catalytic properties of titania–cadmium sulfide hybrid nanostructures. Catal Commun 68:61–66
Huang B, Yang Y, Chen X, Ye D (2010) Preparation and characterization of CdS–TiO2 nanoparticles supported on multi-walled carbon nanotubes. Catal Commun 11:844–847
Liu Y, Zhang P, Tian B, Zhang J (2015) Enhancing the photocatalytic activity of CdS nanorods for selective oxidation of benzyl alcohol by coating amorphous TiO2 shell layer. Catal Commun 70:30–33
Vorokh AS, Kozhevnikova NS, Gorbunova TI, Baklanova IV, Gyrdasova OI, Buldakova LY, Yanchenko MY, Bamburov VG (2016) Mechanism of the formation of photosensitive nanostructured TiO2 with low content of CdS nanoparticles. Dokl Phys Chem 467:56–59
Zyoud AH, Zaatar N, Saadeddin I, Ali C, Park DH, Campet G, Hilal HS (2010) CdS-sensitized TiO2 in phenazopyridine photo-degradation: catalyst efficiency, stability and feasibility assessment. J Hazard Mater 173:318–325
Li X, Wang J, Men Y, Bian Z (2016) TiO2 mesocrystal with exposed (001) facets and CdS quantum dots as an active visible photocatalyst for selective oxidation reactions. Appl Catal B 187:115–121
Liu Z, Fang P, Wang S, Gao Y, Chen F, Zheng F, Liu Y, Dai Y (2012) Photocatalytic degradation of gaseous benzene with CdS-sensitized TiO2 film coated on fiberglass cloth. J Mol Catal A 363–364:159–165
Ghows N, Entezari MH (2011) Exceptional catalytic efficiency in mineralization of the reactive textile azo dye (RB5) by a combination of ultrasound and core–shell nanoparticles (CdS/TiO2). J Hazard Mater 195:132–138
Mancipe S, Tzompantzi F, Gómez R (2017) Photocatalytic reduction of 4-nitrophenol to 4-aminophenol over CdS/MgAl layered double hydroxide catalysts under UV irradiation. Reac Kinet Mech Cat 122:625–634
Wang M, Hua J, Yang Y (2018) Fabrication of CDs/CdS-TiO2 ternary nano-composites for photocatalytic degradation of benzene and toluene under visible light irradiation. Spectrochim Acta A 199:102–109
Song Y, Li N, Chen D, Xu Q, Li H, He J, Lu J (2018) 3D ordered MoP inverse opals deposited with CdS quantum dots for enhanced visible light photocatalytic activity. Appl Catal B 238:255–262
Dong Y-Z, Xue Y-S, Yang W-W, You H-M, Su Y (2019) Visible light driven CdS/WO3 inverse opals with enhanced RhB degradation activity. Colloids Surf A 561:381–387
Mullin MD, Pochini CM, McCrindle S, Romkes M, Safe SH, Safe LM (1984) High-resolution PCB analysis: synthesis and chromatographic properties of all 209 PCB congeners. Environ Sci Technol 18:468–476
Becker H, Berger W, Domschke G, Fanghänel E, Faust J, Fischer M, Gents F, Gewald K, Gluch R, Mayer R, Müller K, Pavel D, Schmidt H, Schollberg K, Schwetlick K, Seiler E, Zeppenfeld G (1967) Organikum. Organisch-chemisches Grundpraktikum, sixth ed., VEB Deuutcher Verlag der Wissenschaften, Berlin
Kozhevnikova NS, Vorokh AS, Rempel AA (2010) Preparation of stable colloidal solution of cadmium sulfide CdS using ethylenediaminetetraacetic acid. Russ J Gen Chem 80:391–394
Vorokh AS, Kozhevnikova NS, Gorbunova TI, Gyrdasova OI, Baklanova IV, Buldakova LY, Yanchenko MY, Murzakaev AM, Shalaeva EV, Enyashin AN (2017) Facile, rapid and efficient doping of amorphous TiO2 by pre-synthesized colloidal CdS quantum dots. J Alloys Compd 706:205–214
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomery JA (1993) General atomic and molecular electronic structure system. J Comput Chem 14:1347–1363
Chana A, Concejero MA, de Frutos M, González MJ, Herradón B (2002) Computational studies on biphenyl derivatives. Analysis of the conformational mobility, molecular electrostatic potential, and dipole moment of chlorinated biphenyl: searching for the rationalization of the selective toxicity of polychlorinated biphenyls (PCBs). Chem Res Toxicol 15:1514–1526
Lopes C, Perga ME, Peretti A, Roger MC, Persat H, Babut M (2011) Is PCBs concentration variability between and within freshwater fish species explained by their contamination pathways? Chemosphere 85:502–508
Da Silva JP, Jockusch S, Turro NJ (2009) Probing the photoreactivity of aryl chlorides with oxygen. Photochem Photobiol Sci 8:210–216
Manzano MA, Perales JA, Sales D, Quiroga JM (2004) Using solar and ultraviolet light to degrade PCBs in sand and transformer oils. Chemosphere 57:645–654
Yao Y, Kakimoto K, Ogawa HI, Kato Y, Kadokami K, Shinohara R (2000) Further study on the photochemistry of non-ortho substituted PCBs by UV irradiation in alkaline 2-propanol. Chemosphere 40:951–956
Yao Y, Kakimoto K, Ogawa HI, Kato Y, Hanada Y, Shinohara R, Yoshino E (1997) Reductive dechlorination of non-ortho substituted polychlorinated biphenyls by ultraviolet irradiation in alkaline 2-propanol. Chemosphere 35:891–2897
Hossain MF, Biswas S, Takahashi T (2009) Study of CdS-sensitized solar cells, prepared by ammonia-free chemical bath technique. Thin Solid Films 518:1599–1602
Lu SY, Wu D, Wang QL, Yan J, Buekens AG, Cen KF (2011) Photocatalytic decomposition on nano-TiO2: Destruction of chloroaromatic compounds. Chemosphere 82:1215–1224
Chang FC, Chiu TC, Yen JH, Wang YS (2003) Dechlorination pathways of ortho-substituted PCBs by UV irradiation in n-hexane and their correlation to the charge distribution on carbon atom. Chemosphere 51:775–784
Miao XS, Chu SG, Xu XB (1999) Degradation pathways of PCBs upon UV irradiation in hexane. Chemosphere 39:1639–1650
Bunce NJ, Bergsma JP, Bergsma MD, De Graff W, Kumar Y, Ravanal L (1980) Structure and mechanism in the photoreduction of aryl chlorides in alkane solvents. J Org Chem 45:3708–3713
Siegman JR, Houser JJ (1982) Photodehalogenation of the monochloro and monofluoroanisoles. J Org Chem 47:2773–2779
Da Silva JP, Vieira Ferreira LF, Machado IF, Da Silva AM (2006) Photolysis of 4-chloroanisole in the presence of oxygen. Formation of the 4-methoxyphenylperoxyl radical. J Photochem Photobiol A 182:88–92
Orvis J, Weiss J, Pagni RM (1991) Further studies on the photoisomerization and hydrolysis of chlorobiphenyls in water. Common ion effect in the photohydrolysis of 4-chlorobiphenyl. J Org Chem 56:1851–1857
Lazzaroni S, Protti S, Fagnoni M, Albini A (2010) Participation of a heterolytic path in the photochemistry of chlorobenzene. J Photochem Photobiol A 210:140–144
Mills SA III, Thal DI, Barney J (2007) A summary of the 209 PCB congener nomenclature. Chemosphere 68:1603–1612
Frame GM (1997) A collaborative study of 209 PCB congeners and 6 Aroclors on 20 different HRGC columns. 2. Semi-quantitative Aroclor congener distributions. Fresenius J Anal Chem 357:714–722
Hillery BR, Girard JE, Schantz MM, Wise SA (1997) Characterization of three Aroclor mixtures using a new cyanobiphenyl stationary phase. Fresenius J Anal Chem 357:723–731
Fischer R, Ballschmiter K (1989) Congener-specific identification of technical PCB mixtures by capillary gas chromatography on a n-octyl-methyl silicone phase (SB-Octyl 50) with electron capture- and mass-selective detection. Fresenius J Anal Chem 335:457–463
Karakotia AS, King JES, Vincenta A, Seala S (2010) Synthesis dependent core level binding energy shift in the oxidation state of platinum coated on ceria–titania and its effect on catalytic decomposition of methanol. Appl Catal A 388:262–271
Oida T, Barr JR, Kimata K, McClure C, Lapeza CR, Hosoya K, Ikegami T, Smith CJ, Patterson DC, Tanaka N (1999) Photolysis of polychlorinated biphenyls on octadecylsilylated silica particles. Chemosphere 39:1795–1807
De Felip E, Ferri F, Lupi C, Trleff NM, Volpi F, di Domenleo A (1996) Structure-dependent photocatalytic degradation of polychlorobiphenyls in a TiO2 aqueous system. Chemosphere 33:2263–2271
Burch R, Flambard AR (1981) Reaction specificity in catalysts reported to exhibit strong metal-support interactions. React Kinet Catal Lett 17:23–28
Wang GZ, Wang YW, Chen W, Liang CH, Li GH, Zhang LD (2001) A facile synthesis route to CdS nanocrystals at room temperature. Mater Lett 48:269–272
Ramaiah KS, Pilkington RD, Hill AE, Tomlinson RD, Bhatnagar AK (2001) Structural and optical investigations on CdS thin films grown by chemical bath technique. Mater Chem Phys 68:22–30
Kozhevnikova NS, Vorokh AS, Uritskaya AA (2015) Cadmium sulfide nanoparticles obtained by chemical bath deposition. Russ Chem Rev 84:225–250
Acknowledgements
The work was supported by Ministry of Education and Science of the Russian Federation (No. 075-00578-19-00, No. 0397-2019-0003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gorbunova, T.I., Kozhevnikova, N.S., Vorokh, A.S. et al. Photolysis of polychlorobiphenyls in the presence of nanocrystalline TiO2 and CdS/TiO2. Reac Kinet Mech Cat 126, 1115–1134 (2019). https://doi.org/10.1007/s11144-019-01543-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-019-01543-7