Abstract
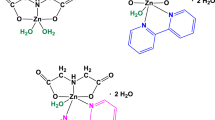
The kinetics and thermodynamics have been studied for the reactions of the copper(II) complexes with iminodiacetate (ida), 2,2′-bipyridine (bipy) and 1,10-phenanthroline (phen) as ligands. The kinetics of substitution reactions of two aqua ligands for bipy and phen in the [Cu(ida)(H2O)2] coordination compound has been studied in water and three type of aqueous solutions of the following surfactants: anionic sodium dodecyl sulfate (SDS), cationic hexadecyl trimethyl-ammonium bromide (CTAB) and nonionic t-octylphenoxypolyetoxyethanol (Triton X-100). The progress of the substitution reactions in the studied solutions was monitored spectrophotometrically using the stopped-flow method. The studies have allowed the determination of the effect of the type of surfactant solutions on the rate of the substitution reaction. Moreover, the order of studied reactions has been determined. The research performed has also allowed us to propose the reaction mechanism of the [Cu(ida)(H2O)2] binary complex with chelate ligands (bipy or phen). In addition, the thermodynamic stability of complexes under study in aqueous solutions has been examined using the potentiometric titration method. Moreover, the potential scavenging activity of the copper(II) complexes has been investigated towards the superoxide radical.




Similar content being viewed by others
References
Roman-Alpiste MJ, Martin-Ramos JD, Castineiras-Campos A, Bugella-Altamirano E, Sicilia-Zafra AG, Gonzalez-Perez JM, Niclos-Gutierrez J (1999) Polyhedron 18:3341–3351
Ren YP, Long LS, Mao BW, Yuan YZ, Huang RB, Zheng LS (2003) Angew Chem Int Ed 115:550–553
Hong-Bin X, Li-Kai Y, Zhong-Min S, Shu-Mei Y, Heng-Już Z, Kui-Zhan S, Ya-Hui Z (2004) Transit Met Chem 29:471–476
Selvakumar B, Rajendiran V, Maheswari PU, Stoeckli-Evans H, Palaniavar M (2006) J Inorg Biochem 100:316–330
Pavlishchuk AV, Kolotilov SV, Zeller M, Thompson LK, Addison AW (2014) Inorg Chem 53:1320–1330
Pranczk J, Jacewicz D, Wyrzykowski D, Tesmar A, Chmurzyński L (2015) J Chem Sci 127:1845–1852
Holyer RH, Hubbard CD, Kettle SDA, Wilkins RG (1956) Inorg Chem 4:929–935
Bunton CA (2006) Adv Colloid Interface Sci 123–136:333–343
Dwars T, Paetzold E, Oehme G (2005) Angew Chem Int Ed 44:7174–7199
Ruiz CC (1995) Colloid Polym Sci 273:1033–1040
Singh A, Van Hamme JD, Ward OP (2007) Biotechnol Adv 25:99–121
Samiey B, Toosi AR (2009) Bull Korean Chem Soc 30:2051–2056
Hodges HL, De Araujo MA (1982) Inorg Chem 21:3236–3239
Bellam R, Anipindi NR (2014) Transit Met Chem 39:311–326
Wang JS, Matyjaszewski K (1995) Macromolecules 28:7901–7910
Fridovich I (1995) Annu Rev Biochem 64:97–112
Harrison PG, Ball IK, Azelee W, Daniell W, Goldfarb D (2000) Chem Mater 12:3715–3725
Siddiqi ZA, Sharma PK, Shahid M, Khalid M, Siddique A, Kumar S (2012) Eur J Med Chem 57:102–111
Siddiqi ZA, Sharma PK, Shahid M, Khalid M, Kumar S (2011) J Mol Struct 994:295–301
Singh V, Tyagi R (2015) J Taibah Univ Sci 9:477–489
Brariz I, Barriada J, Vilarino T, de Vicente MS (2004) Monatsh Chem 135:1475–1488
Chmurzyński L (1996) Anal Chim Acta 326:267–274
Chmurzyński L, Nesterowicz M, Wawrzyniak G, Kaczmarczyk E, Warnke Z (1996) Aust J Chem 49:931–942
Gans P, Sabatini A, Vacca A (1996) Talanta 43:1739–1753
Alderighi L, Gans P, Ienco A, Peters D, Sabatini A, Vacca A (1999) Coord Chem Rev 184:311–318
Pranczk J, Jacewicz D, Wyrzykowski D, Chmurzynski L (2014) Curr Pharm Anal 10:293–304
Audri RL, Allen AO, Bielski BH (1981) FEBS Lett 135:265–267
Pranczk J, Jacewicz D, Wyrzykowski D, Wojtczak A, Tesmar A, Chmurzyński L (2015) Eur J Inorg Chem 20:3343–3349
Wyrzykowski D, Inkielewicz-Stępniak I, Czupryniak J, Jacewicz D, Ossowski T, Woźniak M, Chmurzyński L (2013) Z Anorg Allg Chem 639:1795–1799
Pranczk J, Wyrzykowski D, Jacewicz D, Sikorski A, Tesmar A, Chmurzyński L (2015) Polyhedron 100:74–81
Rosi M, Sgamellotti A, Tarantelli F, Bertini I, Luchinat C (1986) Inorg Chem 25:1005–1008
Wyrzykowski D, Pranczk J, Jacewicz D, Tesmar A, Pilarski B, Chmurzyński L (2014) Cent Eur J Chem 12:107–114
Sillen LG, Martel AE (1966) Stability constants of metal-ion complexes. The Chemical Society, London
Acknowledgements
This work was supported by National Science Centre, Poland under Grant Number 2015/19/N/ST5/00276.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Drzeżdżon, J., Piotrowska, A., Wyrzykowski, D. et al. Kinetics and thermodynamics of the reaction of iminodiacetate copper(II) complexes with 1,10-phenanthroline and 2,2′-bipyridine in aqueous, anionic, cationic and nonionic surfactants solutions. Reac Kinet Mech Cat 122, 729–740 (2017). https://doi.org/10.1007/s11144-017-1269-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-017-1269-9