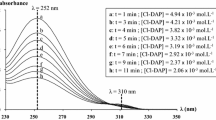
Abstract
Another route to obtain pyrazolidine was developed. It consists of a three step mechanism, which involves, first, the sequential formation of N,N′-dichloro-1,3-diaminopropane starting from 1,3-diaminopropane and sodium hypochlorite, second, a dehydrohalogenation of the dichloro derivative leading to 1-pyrazoline and 2-pyrazoline and finally, the hydrogenation of these two derivatives in order to obtain the compound of interest. In this paper, our study was focused on the kinetics of the formation of 1-pyrazoline and 2-pyrazoline. A kinetic model was proposed and then validated by experimental results. A quantum calculation (Gaussian09/CBS-QB3) was performed in order to determine the stability of the 1 and 2-pyrazolines. It showed that the 2-pyrazoline is more stable than the 1-pyrazoline. This process has been validated on the ISOCHEM production site.











Similar content being viewed by others
References
El Hajj A, Bougrine A-J, Le DM, Pasquet V, Delalu H (2015) A new concept for preparing pyrazolidine by oxidation of 1,3-diaminopropane. Synthesis and formulation of a kinetic model. React Kinet Mech Cat 117(2):429–446
El Hajj A, Delalu H, Bougrine A-J, Philippe C (2014) Process for synthesis of endocyclic hydrazines. International Patent WO2014096042 A1
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09, Revision B.01. Gaussian Inc, Wallingford, CT
Nyden MR, Petersson GA (1981) Complete basis set correlation energies. I. The asymptotic convergence of pair natural orbital expansions. J Chem Phys 75:1843
Petersson GA, Al-Laham MA (1991) A complete basis set model chemistry. II. Open-shell systems and the total energies of the first-row atoms. J Chem Phys 94:6081
Petersson GA, Tensfeldt T, Montgomery JA (1991) A complete basis set model chemistry. III. The complete basis set-quadratic configuration interaction family of methods. J Chem Phys 94:6091
Montgomery JA, Ochterski JW, Petersson GA (1994) A complete basis set model chemistry. IV. An improved atomic pair natural orbital method. J Chem Phys 101:5900
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3093
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El Hajj, A., Bougrine, A.J., Le, D.M. et al. Another route to obtain pyrazolidine passing through the synthesis of 1 and 2-pyrazoline by intramolecular Raschig way. Reac Kinet Mech Cat 120, 619–635 (2017). https://doi.org/10.1007/s11144-016-1117-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-016-1117-3