Abstract
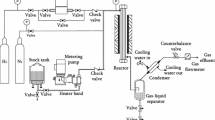
Catalytic hydroprocessing of low temperature coal tar (LTCT) <360 °C distillate was performed over commercial Ni–W/γ-Al2O3, Ni–Mo/γ-Al2O3 and Co–Mo/γ-Al2O3 catalysts in a fixed-bed reactor to produce clean liquid fuel. Experimental feedstock and liquid products were divided into twelve groups based on converted material with the molecules and saturation levels to investigate the effect of catalysts on reaction network of the main compounds in feedstock by GC/MS analysis. Meanwhile, the influence of catalyst component on the product composition like group composition, distribution of carbon number, heteroatom removal, gaseous and oil properties was also investigated. The experimental results showed that phenols were mainly transformed into cycloalkanes and alkylbenzene, while alkyl-naphthalenes were converted to phenyl cycloalkanes and phenyl olefins. In addition, total carbon number in a slightly decreasing tendency. The Ni–W/γ-Al2O3 catalysts showed a higher hydrogenation activity and intermediate carbon number (C9–C16) selectivity, whereas the higher HDN and ring open activity was observed using Ni-Mo/γ-Al2O3 catalyst. The highest percentage of sulfur removal (about 97.8 %) was reached over the Co-Mo/γ-Al2O3 catalyst. Besides, methane was the predominant constituent in the light hydrocarbon gaseous product. And the properties of raw LTCT could be considerably upgraded after hydrotreatment.







Similar content being viewed by others
Abbreviations
- LTCT:
-
Low temperature coal tar
- GC/MS:
-
Gas chromatograph/mass spectrometer
- LHSV:
-
Liquid hourly space velocity
- NIST:
-
Institute of Standards and Technology
- LCA:
-
Long-chain alkanes
- SCA:
-
Short-chain alkanes
- MCA:
-
Monocyclic alkanes
- BCA:
-
Bicyclic alkane
- AC:
-
Alkenes and cycloalkenes
- AB:
-
Alkylbenzenes
- PCA:
-
Phenyl cycloalkanes
- PO:
-
Phenyl olefins
- AN:
-
Alkylnaphthalenes
- P:
-
Phenols
- OC:
-
Other oxygen containings
- FAB:
-
Fluorenes, Anthracenes, Biphenyls
- HDO:
-
Hydrodeoxygenation
- HDS:
-
Hydrodesulfurization
- HDN:
-
Hydrodenitrogeneration
References
Zhufeng Y, Xingzhou Z (2005) Coal market outlook in China. Int J Global Energy Issues 24(3–4):211–227
Li C, Suzuki K (2010) Resources, properties and utilization of tar. Resour Conserv Recycl 54(11):905–915
Xie K, Li W, Zhao W (2010) Coal chemical industry and its sustainable development in China. Energy 35(11):4349–4355. doi:10.1016/j.energy.2009.05.029
Xu J, Yang Y, Li Y-W (2015) Recent development in converting coal to clean fuels in China. Fuel 152:122–130. doi:10.1016/j.fuel.2014.11.059
Edwards JH, Schluter K, Tyler RJ (1986) Upgrading of flash pyrolysis tars to synthetic crude oil: 3. Overall performance of the two-stage hydrotreating process and characterization of the synthetic crude oil. Fuel 65(2):208–211. doi:10.1016/0016-2361(86)90008-6
Kusy J, Andel L, Safarova M, Vales J, Ciahotny K (2012) Hydrogenation process of the tar obtained from the pyrolisis of brown coal. Fuel 101:38–44. doi:10.1016/j.fuel.2011.08.016
Wandas R, Surygala J, Śliwka E (1996) Conversion of cresols and naphthalene in the hydroprocessing of three-component model mixtures simulating fast pyrolysis tars. Fuel 75(6):687–694
Demirel B, Wiser W, Oblad A, Zmierczak W, Shabtai J (1998) Production of high octane gasoline components by hydroprocessing of coal-derived aromatic hydrocarbons. Fuel 77(4):301–311
Herod AA, Stokes BJ, Schulten H-R (1993) Coal tar analysis by mass spectrometry—a comparison of methods. Fuel 72(1):31–43. doi:10.1016/0016-2361(93)90372-9
Wang PF, Jin LJ, Liu JH, Zhu SW, Hu HQ (2013) Analysis of coal tar derived from pyrolysis at different atmospheres. Fuel 104:14–21. doi:10.1016/j.fuel.2010.06.041
Li D, Li Z, Li WH, Liu QC, Feng ZL, Fan Z (2013) Hydrotreating of low temperature coal tar to produce clean liquid fuels. J Anal Appl Pyrol 100:245–252. doi:10.1016/j.jaap.2013.01.007
Wailes PC, Bell AP, Triffett ACK, Weigold H, Galbraith MN (1980) Continuous hydrogenation of Yallourn brown-coal tar. Fuel 59(2):128–132. doi:10.1016/0016-2361(80)90054-X
Wiser WH, Singh S, Qader SA, Hill GR (1970) Catalytic hydrogenation of multiring aromatic coal tar constituents. Indus Eng Chem Product Res Dev 9(3):350–357
Demirel B, Wiser WH (1997) High conversion (98 %) for the hydrogenation of 1-methylnaphthalene to methyldecalins. Fuel Process Technol 53(1–2):157–169. doi:10.1016/S0378-3820(97)00044-1
Gilbert G, Weil RC, Hunter RH (1961) Hydrorefining Coal-Tar Naphthalene. Ind Eng Chem 53(12):993–996. doi:10.1021/ie50624a027
Arribas M, Martınez A (2002) The influence of zeolite acidity for the coupled hydrogenation and ring opening of 1-methylnaphthalene on Pt/USY catalysts. Appl Catal A 230(1):203–217
Gevert B, Otterstedt J, Massoth F (1987) Kinetics of the HDO of methyl-substituted phenols. Appl Catal 31(1):119–131
Rosal R, Diez FV, Sastre H (1992) Catalytic hydrogenation of multi ring aromatic hydrocarbons in a coal tar fraction. Ind Eng Chem Res 31(4):1007–1012
Girgis MJ, Gates BC (1991) Reactivities, reaction networks, and kinetics in high-pressure catalytic hydroprocessing. Ind Eng Chem Res 30(9):2021–2058. doi:10.1021/ie00057a001
Lapinas AT, Klein MT, Gates BC, Macris A, Lyons JE (1991) Catalytic hydrogenation and hydrocracking of fluorene: reaction pathways, kinetics, and mechanisms. Ind Eng Chem Res 30(1):42–50
Li X, Zong ZM, Ma WW, Cao J-P, Mayyas M, Wei ZH, Li Y, Yan HL, Wang D, Yang R, Wei X-Y (2015) Multifunctional and highly active Ni/microfiber attapulgite for catalytic hydroconversion of model compounds and coal tars. Fuel Process Technol 134:39–45. doi:10.1016/j.fuproc.2014.12.004
Lemberton J, Touzeyidio M, Guisnet M (1989) Catalytic hydroprocessing of simulated coal tars: II. Effect of acid catalysts on the hydroconversion of model compounds on a sulphided Ni–Mo/Al2O3 catalyst. Applied catalysis 54(1):101–109
Lemberton J, Touzeyidio M, Guisnet M (1989) Catalytic hydroprocessing of simulated coal tars: I. Activity of a sulphided ni–mo/Al2o3 catalyst for the hydroconversion of model compounds. Applied catalysis 54(1):91–100
Lemberton J, Touzeyidio M, Guisnet M (1991) Catalytic hydroconversion of simulated coal tars: III. Activity of sulphided NiMo on alumina-zeolite catalysts for the hydroconversion of model compounds. Appl Catal A 79(1):115–126
Surygala J, Wandas R, Sliwka E (1994) Hydrogenation of multicomponent mixture simulating fast pyrolysis tar. React Kinet Catal Lett 53(1):217–221
Dai F, Gong M, Li C, Li Z, Zhang S (2015) New kinetic model of coal tar hydrogenation process via carbon number component approach. Appl Energ 137:265–272
Li D, Li W, Cui L, Yang X, Zhang M, Yan S (2011) Optimization of processing parameters and macrokinetics for hydrodenitrogenation of coal tar. Adv Sci Lett 4(4–5):1514–1518
Qader S, Wiser W, Hill G (1968) Kinetics of the Hydroremoval of Sulfur, Oxygen, and Nitrogen from a Low Temperature Coal Tar. Indus Eng Chem Process Des Dev 7(3):390–397
Sun J, Li D, Yao R, Sun Z, Li X, Li W (2015) Modeling the hydrotreatment of full range medium temperature coal tar by using a lumping kinetic approach. Reac Kinet Mech Cat 114(2):451–471
Zhu Y, Zhang Y, Dan Y, Yuan Y, Zhang L, Li W, Li D (2015) Optimization of reaction variables and macrokinetics for the hydrodeoxygenation of full range low temperature coal tar. Reac Kinet Mech Cat 116(2):433–450. doi:10.1007/s11144-015-0900-x
Tang W, Fang MX, Wang HY, Yu PL, Wang QH, Luo ZY (2014) Mild hydrotreatment of low temperature coal tar distillate: Product composition. Chem Eng J 236:529–537. doi:10.1016/j.cej.2013.09.038
Teo KC, Watkinson AP (1990) Product distributions from catalytic hydrotreating of a coal tar middle distillate. Fuel 69(10):1211–1218
Kan T, Wang HY, He HX, Li CS, Zhang SJ (2011) Experimental study on two-stage catalytic hydroprocessing of middle-temperature coal tar to clean liquid fuels. Fuel 90(11):3404–3409. doi:10.1016/j.fuel.2011.06.012
Lei S, Z-h ZHANG, Z-g QIU, Fang G, ZHANG W, ZHAO Lf (2015) Effect of phosphorus modification on the catalytic properties of Mo-Ni/Al2O3 in the hydrodenitrogenation of coal tar. J Fuel Chem Technol 43(1):74–80
Cervantes-Gaxiola ME, Arroyo-Albiter M, Perez-Larios A, Balbuena PB, Espino-Valencia J (2013) Experimental and theoretical study of NiMoW, NiMo, and NiW sulfide catalysts supported on an Al–Ti–Mg mixed oxide during the hydrodesulfurization of dibenzothiophene. Fuel 113:733–743. doi:10.1016/j.fuel.2013.06.041
Liaw S-J, Keogh RA, Thomas GA, Davis BH (1994) Catalytic hydrotreatment of coal-derived naphtha using commercial catalysts. Energy Fuels 8(3):581–587. doi:10.1021/ef00045a011
Ferdous D, Dalai AK, Adjaye J (2006) Comparison of product selectivity during hydroprocessing of bitumen derived gas oil in the presence of NiMo/Al2O3 catalyst containing boron and phosphorus. Fuel 85(9):1286–1297. doi:10.1016/j.fuel.2005.11.018
Legarreta JA, Caballero BM, de Marco I, Chomón MJ, Uría PM (1997) Comparison of the effect of catalysts in coal liquefaction with tetralin and coal tar distillates. Fuel 76(13):1309–1313
Stiegel GJ, Tischer RE, Polinski LM (1983) Hydroprocessing of solvent-refined coal: effect of catalyst properties. Indus Eng Chem Product Research Dev 22(3):411–420
Wen WY, Cain E (1984) Catalytic pyrolysis of a coal tar in a fixed-bed reactor. Indus Eng Chem Product Research Dev 23(4):627–637. doi:10.1021/i200027a001
Šafářová M, Kusý J, Anděl L (2010) Brown coal tar hydrotreatment. J Anal Appl Pyrol 89(2):265–270. doi:10.1016/j.jaap.2010.09.002
Kan T, Sun X, Wang H, Li C, Muhammad U (2012) Production of gasoline and diesel from coal tar via its catalytic hydrogenation in serial fixed beds. Energy Fuels 26(6):3604–3611. doi:10.1021/ef3004398
Gu Z, Chang N, Hou X, Wang J, Liu Z (2012) Experimental study on the coal tar hydrocracking process in supercritical solvents. Fuel 91(1):33–39. doi:10.1016/j.fuel.2011.07.032
Han L, Zhang R, Bi J (2009) Experimental investigation of high-temperature coal tar upgrading in supercritical water. Fuel Process Technol 90(2):292–300
C-x Ma, Zhang R, J-c Bi (2003) Upgrading of coal tar in supercritical water. J Fuel Chem Technol 31(2):103–110
Chang N, Gu Z, Wang Z, Liu Z, Hou X, Wang J (2011) Study of Y zeolite catalysts for coal tar hydro-cracking in supercritical gasoline. J Porous Mat 18(5):589–596
Chareonpanich M, Zhang ZG, Tomita A (1996) Hydrocracking of Aromatic Hydrocarbons over USY-Zeolite. Energy Fuels 10(4):927–931. doi:10.1021/ef950238m
Korre SC, Klein MT, Quann RJ (1995) Polynuclear aromatic hydrocarbons hydrogenation. 1. experimental reaction pathways and kinetics. Ind Eng Chem Res 34(1):101–117. doi:10.1021/ie00040a008
Massoth FE, Politzer P, Concha MC, Murray JS, Jakowski J, Jack S (2006) Catalytic hydrodeoxygenation of methyl-substituted phenols: correlations of kinetic parameters with molecular properties. J Phys Chem B 110(29):14283–14291
Sullivan RF, Egan CJ, Langlois GE (1964) Hydrocracking of alkylbenzenes and polycyclic aromatic hydrocarbons on acidic catalysts. Evidence for cyclization of the side chains. J Catal 3(2):183–195. doi:10.1016/0021-9517(64)90126-5
Sullivan R, Egan CJ, Langlois G, Sieg RP (1961) A new reaction that occurs in the hydrocracking of certain aromatic hydrocarbons. J Am Chem Soc 83(5):1156–1160
Qader SA, Hill GR (1969) Catalytic Hydrocracking. Hydrocracking of a Low Temperature Coal Tar. Industrial & Engineering Chemistry Process Design and Development 8(4):450–455. doi:10.1021/i260032a003
Qader SA, Hill GR (1969) Catalytic hydrocracking. mechanism of hydrocracking of low temperature coal tar. Indus Eng Chem Process Des Dev 8(4):456–461
Ding L, Zheng Y, Zhang Z, Ring Z, Chen J (2007) HDS, HDN, HDA, and hydrocracking of model compounds over Mo-Ni catalysts with various acidities. Appl Catal A 319:25–37. doi:10.1016/j.apcata.2006.11.016
Sun R, Shen S, Zhang D, Ren Y, Fan J (2015) Hydrofining of coal tar light oil to produce high octane gasoline blending components over γ-Al2o3 and η-Al2o3-supported catalysts. Energy Fuels 29(11):7005
Acknowledgments
We gratefully acknowledge the financial support of the Overall Science and Technology Innovation Project of Shaanxi province (2014KTCL01-09) and Research Fund for the Doctoral Program of Higher Education of China (20126101120013) and Scientific Research Project of the Department of Education of Shaanxi province (14JF026, 15JF031).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cui, W., Zheng, H., Niu, M. et al. Product compositions from catalytic hydroprocessing of low temperature coal tar distillate over three commercial catalysts. Reac Kinet Mech Cat 119, 491–509 (2016). https://doi.org/10.1007/s11144-016-1068-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-016-1068-8