Abstract
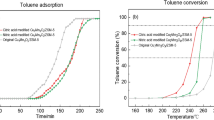
Gas-phase transformation of toluene is carried out on Cs-exchanged NaY zeolite in a fixed-bed tubular reactor and it is observed that toluene can transform through dealkylation, producing benzene and a small amount of hydrogen under the experimental conditions. After a definite activation period, the transformation of toluene can achieve its steady state with the yield of benzene above 25 % for a relatively long continuous time at low space velocity. Combining the results of characterization and catalytic performances, it can be concluded that benzene is the steadiest product except the carbonaceous residues within the CsY zeolite pores. The in situ carbonaceous deposition experiment shows a similar trend of the carbonaceous forming process and the yield of benzene, indicating the important role of carbonaceous species in the formation of benzene, i.e., it is necessary for the accumulation of carbonaceous species in the initial stage of reaction. The discovery in the current work sheds light on new strategies for the transformation of other similar compounds.





Similar content being viewed by others
References
Haber J, Zienkiewicz E (1984) Appl Catal 10:267–271
Doumani TF (1958) Ind Eng Chem 50:1677–1680
Golubyatnikov VI, Kapustin VM, Lugovskoi AI (1987) Chem Technol Fuels Oils 23:418–420
Mavrodinova V, Popova M, Mihályi RM, Pál-Borbély G, Minchev Ch (2004) J Mol Catal A Chem 220:239–246
Al-Khattaf S (2006) Energy Fuels 20:946–954
Huang M, Kaliaguine S (1992) J Chem Soc Faraday Trans 88:751–758
Hunger M, Schenk U, Weitkamp J (1998) J Mol Catal A Chem 134:97–109
Borgna A, Sepúlveda J, Magni SI, Apesteguía CR (2004) Appl Catal A Gen 276:207–215
Borgna A, Magni S, Sepúlveda J, Padró CL, Apesteguía CR (2005) Catal Lett 102:15–21
Zhu JF, Mosey N, Woo T, Huang YN (2007) J Phys Chem C 111:13427–13436
Takeuchi M, Hidaka M, Anpo M (2012) J Hazard Mater 237–238:133–139
Mavrodinova V, Popova M, Mihályi MR, Pál-Borbély G, Minchev C (2004) Appl Catal A Gen 262:75–83
Cecchini JP, Serra RM, Barrientos CM, Ulla MA, Galván MV, Milt VG (2011) Microporous Mesoporous Mater 145:51–58
Frising T, Leflaive P (2008) Microporous Mesoporous Mater 114:27–63
Garcia FAC, Araújo DR, Silva JCM, de Macedo JL, Ghesti GF, Dias SCL, Dias JA, Filho GNR (2011) J Braz Chem Soc 22:1894–1902
Brémard C, Maire ML (1993) J Phys Chem 97:9695–9702
Brémard C, Bougeard D (1995) Adv Mater 7:10–25
Mielczarski E, Davis ME (1990) Ind Eng Chem Res 29:1579–1582
Concepción-Heydorn P, Jia C, Herein D, Pfänder N, Karge HG, Jentoft FC (2000) J Mol Catal A Chem 162:227–246
Acknowledgments
This work is supported by Natural Science Foundation of Jiangsu Province (BK20140191), Postdoctoral Science Foundation of China (2014M551704), the Fundamental Research Funds for the Central Universities (2014QNA05) and A Project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). The help from Professor Weiping Ding of Nanjing University and the support from the Shanghai Research Institute of Petrochemical Technology are also appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, H., Yang, G. Highly selective formation of benzene upon toluene transformation on CsY zeolite. Reac Kinet Mech Cat 113, 605–614 (2014). https://doi.org/10.1007/s11144-014-0759-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-014-0759-2