Abstract
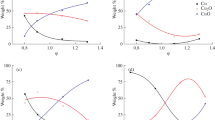
The kinetics of the catalytic soot combustion was studied for each identified stage of structural transformations of the CuMoO4 catalyst. The formation of the catalytically active phase Cu4−xMo3O12 during the process through the redox transformation of the catalyst is controlled by the reduction of CuMoO4 on the catalyst surface by the soot. The oxidation of the soot by the active phase of the catalyst could be described by the Avrami-Erofeev equation for the three-dimensional growth of randomly formed combustion centers. The quantity of the Cu4−xMo3O12 molybdate on the catalyst surface remains constant throughout the catalytic reaction. A macrokinetic model equation describing dependence of the total reaction rate from the rates of redox catalyst transformation and catalytic soot oxidation steps was obtained.









Similar content being viewed by others
References
Neumann B, Kröger C, Fingas E (1931) Zusätzen Zeitschr Anorg Allg Chem 197:321
Shimizu K, Kawachi H, Satsuma A (2009) Appl Catal B 91:489
Sarbak Z, Surma K (2003) JTAC 72:159
Neeft JPA, Makkee M, Moulijn JA (1996) Appl Catal B 8:57
Neeft JPA, van Pruissen OP, Makkee M, Moulijn JA (1997) Appl Cat B 12:21
Machida M, Murata Yu, Kishikawa K, Zhang D, Ikeue K (2008) Chem Mater 20:4489
Krishna K, Bueno-Lo´pez A, Makkee M, Moulijn JA (2007) Appl Catal B 75:189
Gong C, Song Ch, Pei Y, Lv G, Fan G (2008) Ind Eng Chem Res 47:4374
T. Seiyama, Catal Rev—Sci Eng 34 (1992) 281
Hernandez S, Blengini GA, Russo N, Fino D (2012) Ind Eng Chem Res 51:7584
Peng X, Lin H, Shangguan ZW (2006) Ind Eng Chem Res 45:8822
Saab E, Aouad S, Abi-Aad E, Bokova MN, Zhilinskaya EA, Aboukaïs A (2007) Kinet Catal 48(6):841
Bassou B, Guilhaume N, Lombaert K, Mirodatos C, Bianchi D (2010) Energy&Fuels 24:4766
Chigrin PG, Lebukhova NV, Ustinov AYu (2013) Kinet Catal 54(1):76
Neeft JPA (1995) Catalytic oxidation of soot. Ph.D. thesis, Delft University of Technology Delft, p 247
Stanmore BR, Brilhac JF, Gilot P (2001) Carbon 39:2247
Ya. Shestak (1987) Theory of thermal analysis: physical and chemical properties of solid inorganic substances, translated from English, Mir, Moscow, p 456
Brown WE, Dollimore D, Galwey AK in: C.H. Bamford, C.F.H. Tipper (Eds.) (1980) Comprehensive chemical kinetics. Reactions in the solid state, Elsevier, Amsterdam, p 340
Johnson WA, Mehl RF (1939) Trans Inst Ming Metall Engrs 135:416
Machej T, Ziółkowski J (1980) J Solid State Chem 31:145
Zhilenko MP, Rudenko AP, Sivtseva AV (2001) Mosc Univ Chem Bull 42(6):394
Haber J, Machej T, Ungier L, Ziółkowski J (1978) J Solid State Chem 25:207
Kissinger HE (1957) Analyt Chem 29:1702
Bokova MN, Decarne C, Abi-Aad E, Pryakhin AN, Lunin VV, Aboukaïs A (2005) Thermochim Acta 428:165
Zouaoui N, Issa M, Kehrli D, Jeguirim M (2012) Catal Today 189:65
Helfferich FG in: R.G. Compton, G. Hancock (Eds.) 2004 Comprehensive chemical kinetics. Kinetic of multistep reactions, second ed., Elsiever, Amsterdam, p 410
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chigrin, P.G., Lebukhova, N.V. & Ustinov, A.Y. Kinetics of soot oxidation catalyzed by CuMoO4 . Reac Kinet Mech Cat 113, 1–17 (2014). https://doi.org/10.1007/s11144-014-0754-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-014-0754-7