Abstract
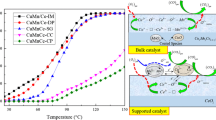
In this study, Cu–Mn composite catalysts were prepared by the slurry coating method using honeycomb cordierite as a support and TiO2 as a carrier. The influence of the relative composition of Cu, Mn on the DeNOx performance was studied for different preparation processes. It was found that the catalyst prepared by adding 15 wt% Mn and 5 wt% Cu into the slurry at the same time has higher conversion than that of the catalyst only using Mn as the active component. For the composite catalyst prepared by loading Mn and Cu onto TiO2 followed by the complex of MnOx/TiO2 and CuOx/TiO2 (weight ratio of 2:1) powder, a high activity is achieved in a temperature range, which can be due to the weak interaction between Cu and Mn. The considerable denitrification of the catalyst should be attributed to the large surface area and pore volume, uniform distribution, higher activity Mn in the form of MnO2 and synergy of Mn, Cu and carrier. The catalyst prepared by two methods were characterized by XRD, XPS, SEM and H2 temperature-programmed reduction (H2-TPR). H2-TPR results reveal that the interaction between Cu and Mn varies with the change of the composition of two components. XPS results illustrated that the relative atomic percentage values of n(Mn4+)/n(Mn3+) and n(Mn4+)/n(Mnn+) were significantly high for the catalyst with better catalytic performance and the MnO2 is the dominant phase with respect to the Mn2O3 and MnO phase in all catalysts. Meanwhile, we can draw the conclusion that the MnCu/ZTC catalyst by the mixed loading way is superior to TMnCu/CC through the order loading method by XPS and NH3-SCR test.









Similar content being viewed by others
References
Ye D (1999) Pollution control of NOx in smoke. Environ Prot Sci 04:1–4
Zhang Y, Shen J, Zhang Z (2007) A new process to prepare supported coupled-catalyst of V2O5/TiO2 used in SCR reaction of NOx. Chin J Rare Met 31(1):105–109
Chen X, Zhong Z (2010) Research progress in the De-NOx catalyst supported on TiO2. Jiangsu Electr Eng 29(2):54–56
Liu Q, Liu Z, Fan J (2005) Selective catalytic reduction of NO by NH3 on CuO catalyst supported on honeycomb-like cordierite coated with Al2O3. Chin J Catal 26(1):59–64
Valdes-Solis T, Marban G, Fuertes AB (2001) Low-temperature SCR of NOx with NH3 over carbon-ceramic cellular monolith-supported manganese oxides. Catal Today 69(1–4):259–264
Valdes-Solis T, Marban G, Fuertes AB (2003) Low-temperature SCR of NOx with NH3 over carbon-ceramic supported catalysts. Appl Catal B Environ 46(2):261–271
Tang X, Hao J, Yi H et al (2007) Low-temperature SCR of NO with NH3 over AC/C supported manganese-based monolithic catalysts. Catal Today 126(3–4):406–411
Blanco J, Avila P, Suárez S et al (2004) CuO/NiO monolithic catalysts for NOx removal from nitric acid plant flue gas. Chem Eng J 97:l–9
Ouzzine M, Cifredo GA, Gatica JM et al (2008) Original carbon-based honeycomb monoliths as support of Cu or Mn catalysts for low-temperature SCR of NO: Effects of preparation variables. Appl Catal A 342(1–2):150–158
Lin T, Zhang Q, Li W et al (2008) Monolith manganese-based catalyst supported on ZrO2–TiO2 for NH3-SCR reaction at low temperature. Acta Physico Chimica Sinica 24(7):1127–1131
Thirupathi B, Smirniotis PG (2012) Nickel-doped Mn/TiO2 as an efficient catalyst for the low-temperature SCR of NO with NH3: Catalytic evaluation and characterizations. J Catal 288:74–83
Kim YJ, Kwon HJ, Heo I, Nam I-S, Cho BK, Choung JW, Cha M-S, Yeo GK (2012) Mn–Fe/ZSM5 as a low-temperature SCR catalyst to remove NOx from diesel engine exhaust. Appl Catal B 126:9–21
Jemal J, Tounsi H, Djemel S, Pettito C, Delahay G (2013) Characterization and deNOx activity of copperhydroxyapatite catalysts prepared by wet impregnation. Reac Kinet Mech Cat 109:159–165
Pena DA, Uphade BS, Smirniotis PG (2004) TiO2-supported metal oxide catalysts for low-temperature selective catalytic reduction of NO with NH3 I. Evaluation and characterization of first row transition metals. J Catal 221(2):421–431
Zou Z-Q, Meng M, Guo L-H et al (2008) Synthesis and characterization of CuO/Ce1−xTixO2 catalysts used for low-temperature CO oxidation. J Hazard Mater 163:835–842
Ettireddy PR, Etireddy N, Mamedov S et al (2007) Surface characterization studied of TiO2 supported manganese oxide catalysts for low temperature SCR of NO with NH3. Appl Catal B 76:123–134
Pena DA, Uphade BS, Reddy EP et al (2004) Identification of surface species on titania-supported manganese, chromium, and copper oxide low-temperature SCR catalysts. J Phys Chem B 108:9927–9936
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, G., Li, Y., Wang, Y. et al. DeNOx performance of Cu–Mn composite catalysts prepared by the slurry coating method. Reac Kinet Mech Cat 111, 235–245 (2014). https://doi.org/10.1007/s11144-013-0614-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-013-0614-x