Abstract
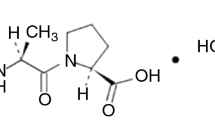
The presented study aimed at the evaluation of the chemical stability properties of cilazapril during isothermal kinetic tests, subsequent identification of the degradation product and proposition of a probable mechanism of cilazapril degradation. Identification analyses gave undeniable evidence that cilazapril degrades in course of deesterification reaction forming cilazaprilat. This transformation has negative implications, since cilazaprilat, although biologically active, is not capable of being absorbed from gastrointestinal tract. Thermal analyses pointed out that the kinetic model of cilazapril degradation in solid state depends on the presence of moisture in the surrounding environment. Under the conditions of increased relative humidity levels, changes in cilazapril concentration during the degradation process can be expressed by the Prout–Tompkins relationship. The decomposition process occurs more rapidly, while increasing both the relative humidity (lnk = (0.036 ± 0.006) RH - (13.53 ± 0.23)) and the temperature (lnk = (−20025.3 ± 2500.3) 1/T + (44.21 ± 7.19)). On the other hand, the lack of moisture in air generates a change in the kinetic model of reaction. In the absence of humidity, a different reaction model is suggested with two reaction stages. Here the increase of temperature also affects both stages of the reaction in linear semi-logarithmic way (lnk 1 = (−10394.4 ± 1976.8) 1/T + (15.15 ± 5.18) and lnk 2 = (−13255.9 ± 2679.2) 1/T + (20.82 ± 7.02)). The obtained results enabled a comparison with other angiotensin enzyme inhibitors similar in structure, which showed that cilazapril in the solid state is characterized by the best chemical stability (E a = 166.50 ± 20.79 kJ mol−1).










Similar content being viewed by others
References
Council of Europe (2010) European directorate for the quality of medicines and healthcare. European Pharmacopoeia, Strasbourg
Opie LH (1999) Angiotensin-converting enzyme inhibitors. The advance continues. Authors’ Publishing House, New York
Attwood MR (1989) Br J Clin Pharmacol 27(Suppl 2):133S–137S
Waterfall JF (1989) Br J Clin Pharmacol 27(Suppl 2):139S–150S
Szucs T, Schneeweiss A (1992) Cardiology 80:34–41
Byrn SR, Xu W, Newman AW (2001) Adv Drug Deliv Rev 48:115–136
Yoshioka S, Stella VJ (2002) Stability of drugs and dosage forms. Kulwer Academic Publishers, New York
Stanisz B (2003) J Pharm Biomed Anal 31:375–380
Stanisz B (2003) Acta Pol Pharm 60:443–450
Stanisz B (2004) J Liq Chromatogr Relat Tech 27:3103–3119
Stanisz B (2004) Acta Pol Pharm 61:91–97
Paszun SK, Stanisz BJ, Pawłowski W (2012) Acta Pol Pharm 70:193–201
Brown ME (1997) Thermochim Acta 30:93–106
Pawełczyk E, Hermann T (1982) Podstawy trwałości leków (Principles of drug stability). PZWL, Warsaw
Acknowledgments
The research was funded by the Polish National Science Centre (Grant No. NN405 050440).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paszun, S.K., Stanisz, B.J. Cilazapril decomposition kinetics and mechanism in the solid state versus stability of the other ester pro-drug angiotensin converting enzyme inhibitors. Reac Kinet Mech Cat 109, 285–300 (2013). https://doi.org/10.1007/s11144-013-0558-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-013-0558-1