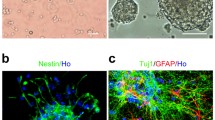
We present the results of studying the impact of high-intensity terahertz pulses on the differentiation of induced human neural progenitor cells (drNPCs). The differentiation was estimated using the immunocytochemical analysis, i.e., the marker of the undifferentiated cells (SOX2) and the markers of the neuronal (β-III-tubulin and MAP2b) and glial (GFAP) phenotype. The cell exposure was performed by terahertz pulses with an intensity of 21 GW/cm2 and an electric field of 2.8 MV/cm for 30 min. As a result of exposure, the phenotype of induced neural progenitor cells did not differ from that of unexposed cells and the appearance of mature neurons or glial cells was not detected. The ability of the terahertz radiation to cause effects in neural cell cultures apparently requires further studies for higher exposure intensity or duration.
Similar content being viewed by others
References
I. V. Il’ina, D. S. Sitnikov, and M. B. Agranat, High Temp., 56, No. 5, 789–810 (2018). https://doi.org/10.1134/S0018151X18050127
O.P.Cherkasova, D. S. Serdyukov, A. S.Ratushnyak, et al., Opt. Spectrosc., 128, No. 6, 855–866 (2020). https://doi.org/10.1134/S0030400X20060041
O.P.Cherkasova,D. S. Serdyukov, E. F.Nemova, et al., J. Biomed. Opt., 26, No. 09, 090902 (2021). https://doi.org/10.1117/1.jbo.26.9.090902
A. I. Nikitkina, P. Y. Bikmulina, E.R. Gafarova, et al., J. Biomed. Opt., 26, No. 4, 043005 (2021). https://doi.org/https://doi.org/10.1117/1.jbo.26.4.043005
N.P. Bondar, I. L.Kovalenko, D. F.Avgustinovich, et al., Bull. Exp. Biol. Med. 145, No. 4, 401–405 (2008). https://doi.org/10.1007/s10517-008-0102-x
V.F.Kirichuk, N.V. Efimova, and E.V.Andronov, Bull. Exp. Biol. Med., 148, No. 5, 746–749 (2009). https://doi.org/https://doi.org/10.1007/s10517-010-0807-5
Yu.Ol’shevskaya, A. S.Kozlov, A. K.Petrov, et al., I. P. Pavlov Zh. Vyssh. Nerv. Deyat., 59, No. 3, 353–359 (2009).
N. Bourne, R.H.Clothier, M.DÁrienzo, and P. Harrison, Altern. Lab. Anim., 36, No. 6, 667–684 (2008). https://doi.org/10.1177/026119290803600610
T.Tachizaki, R. Sakaguchi, S.Terada, et al., Opt. Lett., 45, No. 21, 6078 (2020). https://doi.org/10.1364/OL.402815
J.-E.Ahlfors, A.Azimi, R. El-Ayoubi, et al., Stem Cell Res. Ther., 10, No. 1, 166 (2019). https://doi.org/https://doi.org/10.1186/s13287-019-1255-4
V.P.Baklaushev, O.V.Durov, V.A.Kalsin, et al., World J. Stem Cells, 13, No. 5, 452–469 (2021). https://doi.org/10.4252/wjsc.v13.i5.452
K.H.Yang, P. L.Richards, and Y.R. Shen, Appl. Phys. Lett., 19, No. 9, 320–323 (1971). https://doi.org/https://doi.org/10.1063/1.1653935
G. Krizs´an, Z. Tibai, G.T´oth, et al., Opt. Express, 30, No. 3, 4434–4443 (2022). https://doi.org/10.1364/oe.440883
X.Wu, J.Ma, B. Zhang, et al., Opt. Express, 26, No. 6, 7107–7116 (2018). https://doi.org/https://doi.org/10.1364/OE.26.007107
H.Hirori, A.Doi, F.Blanchard, and K.Tanaka, Appl. Phys. Lett., 98, No. 9, 091106 (2011). https://doi.org/https://doi.org/10.1063/1.3560062
M. Shalaby and C.P.Hauri, Nat. Commun., 6, No. 6, 6 (2015). https://doi.org/https://doi.org/10.1038/ncomms6976
P. Liu, X. Zhang, C.Yan, et al., Appl. Phys. Lett., 108, No. 1, 011104 (2016). https://doi.org/10.1063/1.4939456
A. Schneider, M.Neis, M. Stillhart, et al., J. Opt. Soc. Am. B, 23, No. 9, 1822–1835 (2006). https://doi.org/https://doi.org/10.1364/JOSAB.23.001822
C. Vicario, M. Jazbinsek, A. V.Ovchinnikov, et al., Opt. Express, 23, No. 4, 4573–4580 (2015). https://doi.org/10.1364/OE.23.004573
A. V.Ovchinnikov, O. V.Chefonov, D. S. Sitnikov, et al., Quantum Electron., 48, No. 6, 554–558 (2018). 101070/QEL_16681
D. S. Sitnikov, S.A.Romashevskiy, A.V. Ovchinnikov, et al., Laser Phys. Lett., 16, No. 11, 115302 (2019). https://doi.org/https://doi.org/10.1088/1612-202X/ab4d56
D. S. Sitnikov, I.V. Ilina, V. A.Revkova, et al., High Temp., 58, No. 1, 36–43 (2020). https://doi.org/10.1134/S0018151X20010174
D.S.Sitnikov, I.V. Ilina, and A.A. Pronkin, Opt. Eng., 59, No. 6, 061613 (2020). https://doi.org/https://doi.org/10.1117/1.OE.59.6.061613
L. V. Titova, A.K.Ayesheshim, A. Golubov, et al., Sci. Rep., 3, 2363 (2013). https://doi.org/https://doi.org/10.1038/srep02363
L. V. Titova, A.K.Ayesheshim, A. Golubov, et al., Biomed. Opt. Express, 4, No. 4, 559–568 (2013). https://doi.org/https://doi.org/10.1364/BOE.4.000559
D. S. Sitnikov, I.V. Il’ina, S. A. Gurova, et al., Bull. Russ. Acad. Sci. Phys, 84, No. 11, 1370–1374 (2020). https://doi.org/10.3103/1062873820110246
D.S.Sitnikov, V.A.Revkova, I.V. Ilina, et al., J. Biophoton., 16, No. 1, e202200212 (2023). https://doi.org/https://doi.org/10.1002/jbio.202200212
D. S. Sitnikov, A.A.Pronkin, I.V. Ilina, et al., Sci. Rep., 11, 17916 (2021). https://doi.org/https://doi.org/10.1038/s41598-021-96898-0
D. Sitnikov, V.Revkova, I. Ilina, et al., Int. J. Mol. Sci., 24, No. 7, 6558 (2023). https://doi.org/https://doi.org/10.3390/ijms24076558
V.P. Baklaushev, V.G.Bogush, V.A.Kalsin, et al., Sci. Rep., 9, No. 1, 3161 (2019). https://doi.org/10.1038/s41598-019-39341-9
V. A. Revkova, K. V. Sidoruk, V. A.Kalsin, et al., ACS Omega, 6, No. 23, 15264–15273 (2021). https://doi.org/10.1021/acsomega.1c01576
C. M. Hough, D. N. Purschke, C.Bell, et al., Biomed. Opt. Express, 12, No. 9, 5812–5828 (2021). https://doi.org/10.1364/BOE.433240
X. Zhao, M. Zhang, Y. Liu, et al., Science, 24, No. 12, 103485 (2021). https://doi.org/https://doi.org/10.1016/j.isci.2021.103485
S. Ma, Z. Li, S. Gong, et al., Brain Sci., 13, No. 4, 686 (2023). https://doi.org/https://doi.org/10.3390/brainsci13040686
S. Ma, Z. Li, S. Gong, et al., Front. Bioeng. Biotechnol., 11, 1147694 (2023). https://doi.org/https://doi.org/10.3389/fbioe.2023.1147684
J.-E.Ahlfors and R.Elayoubi, “Methods for reprogramming cells and uses thereof,” Canada Patent No.CA-2779310-A1 (2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Izvestiya Vysshikh Uchebnykh Zavedenii, Radiofizika, Vol. 66, Nos. 7–8, pp. 683–696, April 2023. Russian https://doi.org/10.52452/00213462_2023_66_07_683
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sitnikov, D.S., Revkova, V.A., Ilina, I.V. et al. Influence of High-Intensity Terahertz Radiation on the Differentiation of Human Neural Progenitor Cells. Radiophys Quantum El (2024). https://doi.org/10.1007/s11141-024-10321-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11141-024-10321-y