Abstract
Purpose
The impact of pediatric traumatic brain injury (pTBI) on health-related quality of life (HRQoL) in children and adolescents remains understudied. Short scales have some advantages in terms of economy and administration over longer scales, especially in younger children. The aim of the present study is to psychometrically evaluate the six-item German version of the QOLIBRI-OS-KID/ADO scale for children and adolescents. In addition, reference values from a general German pediatric population are obtained to assist clinicians and researchers in the interpretation of HRQoL after pTBI.
Methods
A total of 297 individuals after TBI and 1997 from a general population sample completed the questionnaire. Reliability, validity, and comparability of the assessed construct were examined.
Results
The questionnaire showed satisfactory reliability (α = 0.75 and ω = 0.81 and α = 0.85 and ω = 0.86 for the TBI and general population samples, respectively). The QOLIBRI-OS-KID/ADO was highly correlated with its long version (R2 = 67%) and showed an overlap with disease-specific HRQoL (R2 = 55%) in the TBI sample. The one-dimensional factorial structure could be replicated and tested for measurement invariance between samples, indicating a comparable HRQoL construct assessment. Therefore, reference values and cut-offs indicating clinically relevant impairment could be provided using percentiles stratified by factors significantly associated with the total score in the regression analyses (i.e., age group and gender).
Conclusion
In combination with the cut-offs, the QOLIBRI-OS-KID/ADO provides a cost-effective screening tool, complemented by interpretation guidelines, which may help to draw clinical conclusions and indications such as further administration of a longer version of the instrument to gain more detailed insight into impaired HRQoL domains or omission of further steps in the absence of an indication.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pediatric traumatic brain injury (pTBI) is a global health problem affecting millions of children and adolescents worldwide [1]. It can have a profound impact on multiple aspects of a young individual’s life, including their physical, cognitive, emotional, and social well-being [2]. These outcomes are directly related to health-related quality of life (HRQoL), which remains understudied in pTBI patients [3].
To date, only generic HRQoL measures have been available for use in the pTBI context. However, such generic measures, while useful for comparisons between different disease populations [4], have been found to be too general to sensitively capture the outcome of a specific health condition [5]. Disease-specific HRQoL instruments, on the other hand, provide a more comprehensive assessment by capturing the individual aspects of life affected by a particular disease [6]. Until now, HRQoL after pTBI has been assessed using either generic instruments such as the Pediatric Quality of Life Inventory [7] as a self-report measure, or, more recently, the disease-specific Traumatic Brain Injury Quality of Life Communication Item Bank [8] using the parent perspective, which covers however functional communication only. Given the advantages of disease-specific measures over generic ones, the ability of children to evaluate their own HRQoL [9], and the fact that parent–child assessments of HRQoL are not always congruent [10,11,12], age-appropriate TBI-specific patient-reported outcome measures (PROM) are needed.
To fill this gap, the 35-item self-report Quality of Life after Brain Injury for children and adolescents (QOLIBRI-KID/ADO) questionnaire was developed as the first instrument to assess HRQoL in German-speaking children and adolescents after pTBI [13, 14]. It corresponds to the 37-item adult version (but omits items on satisfaction with sexual life and finances) [15], covers six domains (Cognition, Self, Daily Life & Autonomy, Social Relationships, Emotions, and Physical Problems) that are particularly relevant for monitoring after (p)TBI [15], and aims to assess TBI-specific HRQoL in the pediatric context and to measure the burden of pTBI across the lifespan.
One of the notable advantages of short scales is their economy and ease of administration. These features are particularly important when working with pediatric populations, as their attention span and fatigue tolerance may be limited in general and after pTBI in particular [2]. The development and use of a short overall scale (i.e., QOLIBRI-OS-KID/ADO) may therefore facilitate the collection of information and allow for administration after more severe pTBI without placing additional burden on the young patients. The six-item adult version has already demonstrated robust psychometric properties [16] and high sensitivity to different patient groups [17], making it an attractive tool in both clinical and research settings. The applicability of the overall scale in adults underscores the potential of a similar measure for children and adolescents. By utilizing a short and valid instrument such as the six-item QOLIBRI-OS-KID/ADO questionnaire, clinicians can efficiently assess the comprehensive aspects of HRQoL dimensions in pTBI patients in a condensed form (i.e., by rating overall satisfaction within each domain), thereby optimizing resources and minimizing patient burden.
The availability of reference values from comparable general populations facilitates the interpretation of questionnaire scores obtained from individual patients. A comparison of the level of HRQoL after pTBI with the level of HRQoL in the general pediatric population will provide a more realistic assessment of the post-injury burden.
With these considerations in mind, this study aims to investigate the psychometric properties and the applicability of the recently developed German six-item QOLIBRI-OS-KID/ADO questionnaire. The objectives are to examine the psychometric properties within the framework of classical test theory and to establish reference values based on a German general population sample of children and adolescents.
Methods
Participants
pTBI sample
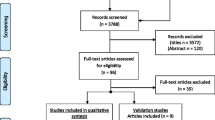
Individuals after pTBI were recruited based on administrative databases of 12 medical centers in Germany and Austria between February 2021 and February 2022. Medical records of potential participating children and adolescents were retrieved from the databases and screened for eligibility. Participants were eligible to participate if they were 8–17 years of age, had been diagnosed with pTBI three months to ten years prior to enrollment, had information on pTBI severity (either based on Glasgow Coma Scale score [18] or medical reports), and were able to understand and respond to questions in German. Children and adolescents with pre-injury epilepsy, very severe polytrauma, severe premorbid mental illness, or life-threatening health conditions were not eligible to participate. Mail invitations were sent to the parents or legal guardians of children and adolescents who met the inclusion criteria. These invitations explained the purpose of the study, the setting, and contact information for study participation. Written informed consent was obtained from all participants and/or legal guardians at the time of study enrollment if parents consented to participate. Interviews with children and adolescents were conducted face-to-face, with the assistance of trained investigators, at separately arranged appointments (online or on-site). Parents completed paper–pencil versions of the questionnaires. A priori power analyses indicated that the primary objective of the project (i.e., to develop the long version of the TBI-specific HRQoL instrument for children and adolescents and to test its psychometric properties) would require a sample of approximately 140 individuals per age group [19]. A total of 297 children and adolescents were included in the analyses (Fig. 1a).
General population sample
Individuals from general population were recruited via an online survey between March 2022 and April 2022 with the assistance of two international market research agencies based in Germany (Dynata: https://www.dynata.com; respondi: https://www.respondi.com; last accessed 05.07.2024). The agencies used their databases to identify and contact parents of children and adolescents aged 8–17 years. Parents were first informed of the purpose of the data assessment and the privacy policy and were asked to provide consent to assessing of sensitive information (i.e., their children’s health data). They were asked if their child had experienced pTBI or any other life-threatening condition. If they responded affirmatively, study participation was terminated. Otherwise, they continued to answer sociodemographic questions and were then asked about their child’s availability. If the child was available, they were invited to participate, or the survey could be continued later. As an incentive, participants received tokens or certificates. The planned sample size was N = 2000 with an even distribution of children and adolescents to obtain robust reference values, taking into account that stratification by other characteristics such as gender may be necessary.
To ensure the data quality, participants who provided inconsistent or unusable information (e.g., conflicting statements of being both completely satisfied and completely bothered), those with contradictory responses (e.g., reporting no health problems but providing comments in the text box), and those who completed the survey in less than five minutes were excluded. A total of 1997 children and adolescents were included in the analyses (Fig. 1b).
Instruments
The Quality of life after brain injury for children and adolescents – overall scale (QOLIBRI-OS-KID/ADO) is a six-item PROM that assesses the overall satisfaction and corresponds to the adult version [16]. The items relate to five out of the six domains (i.e., Physical problems, Cognition, Emotions, Autonomy, Social aspects) of the 35-item version, the QOLIBRI-KID/ADO [13, 14], supplemented by a question on Future prospects. Answers are to be rated on a five-point Likert-type scale (from “Not at all” to “Very”) with a two-week recall period. The scale is accompanied by smileys, which make it easier to rate, especially for younger children. The total score is calculated as the mean of the item responses, transformed into a scale ranging from 0 (lowest possible HRQoL) to 100 (highest possible HRQoL). The latter is done by subtracting 1 from each item score and multiplying by 25. Supplementary material provides information on the development of the questionnaire and the final wording (Development of the QOLIBRI-OS-KID/ADO and Table S1). For administration to the general population, the reference to injury was removed from the instructions. Table 1 summarizes information on further instruments administered in the study.
Sociodemographic and health-related information
In both samples, sociodemographic information included age, gender, and school attendance. In the pTBI sample, health-related information included parent-reported health status (presence of chronic health conditions, neurological disorders, and/or developmental health conditions), TBI severity (mild, moderate, severe), time since injury in years, and functional recovery status assessed by the King’s Outcome Scale for Childhood Head Injury (KOSCHI) [27] with the following categories: (3a/b) lower/upper severe disability, (4a/b) lower/upper moderate disability, and (5a/b) good/intact recovery.
In the general population sample, health status information was reported by parents on nine health domains: central nervous system disease, alcohol and/or psychotropic drug abuse, active or uncontrolled systemic disease, psychiatric disturbances, severe sensory disturbances, use of psychotropic or other drugs, intellectual disability or other neurobehavioral disturbances, problems before, during, and after childbirth, and other. A participant was considered to have at least one chronic condition if at least one domain was endorsed.
Statistical analyses
To ensure comparability of the results of the QOLIBRI-OS-KID/ADO with its long version, we largely followed the analysis scheme of the QOLIBRI-KID/ADO study [13]. For descriptive analyses at the item level, we calculated mean (M), standard deviation (SD), skewness (SK), and frequency of item responses. Skewness was classified as absent (− 0.5 and 0.5), moderate (± 0.5 and ± 1), and high (> ± 1) [28]. For the QOLIBRI-OS-KID/ADO total score, we reported the M, SD, median (Mdn) and quartiles (Q1 and Q3).
Differential item functioning (DIF) analyses using a combination of ordinal logistic regression and item response theory (LORDIF) were conducted to investigate whether the aggregation of data from children (aged 8–12 years) and adolescents (aged 13–17 years) is appropriate. For each item, two LORDIF models were compared separately in the pTBI and the general population samples: Model 1 including ability (i.e., the level of the latent trait, HRQoL, measured by an item), and Model 2 including ability, age category, and the interaction between age category and ability. DIF was considered absent if a non-significant difference (p > 0.01) was found and the effect measured by McFadden’s pseudo R2 was < 0.05 [29]. In the absence of DIF, psychometric analyses can be conducted using aggregated data from children and adolescents.
The reliability was examined in both samples using Cronbach’s α and McDonald’s ω (for both characteristics, values ≥ 0.70 were considered desirable [30]). The reporting of McDonald’s ω, which is calculated based on the factorial structure of a measure and thus accounts for variation in the correlation strength between items and the latent factor [31], was chosen to further support the internal consistency results. We calculated Cronbach’s α while omitting each of the six items to ensure that the scale reliability did not increase when one of the items was removed. Associations between the items and the total score were explored using corrected item-total correlations (CITC > 0.40) [32]. Test–retest reliability was examined in a subsample of patients who completed the questionnaire on two occasions, 10 to 20 days apart. For this purpose, the intraclass correlation coefficient using a two-way random effects model (i.e., ICC(2,1)) was calculated on the total score level, with ICC > 0.60 [33] considered desirable. The standard error of measurement (SEm) [34] and the minimal detectable change (MDC) were calculated based on the ICCs to provide information on the score variability and the “true” change in the QOLIBRI-OS-KID/ADO total score.
Factorial validity was examined using confirmatory factor analysis (CFA) with the weighted least square (WLS) estimator in both the pTBI and the general population samples. The one-factor solution was considered to describe the data well if the goodness-of-fit indices met the following criteria: non-significant χ2-value (p ≥ 0.05) [35], ratio of χ2 and degrees of freedom (df) ≤ 2 [35], comparative fit index (CFI) and Tucker-Lewis index (TLI) > 0.95 [36], root mean square error of approximation (RMSEA) < 0.05 and narrow confidence interval (CI) for an excellent to close fit [37] (fair fit at values of 0.06–0.07, mediocre fit at values of 0.08–0.10, and poor fit at values above 0.10 [36]), and standardized root mean square residual (SRMR) < 0.05 [36]. Scaled measures were reported for all indices except SRMR (not available). Given the potential limitations of interpreting fit indices using the WLS estimator [38], we additionally reported fit indices obtained from the models using robust maximum likelihood (MLR).
Pearson correlation analyses were utilized to investigate convergent and divergent validity through correlations between the QOLIBRI-OS-KID/ADO total score and the questionnaires measuring symptom burden in the pTBI sample. We hypothesized strong positive correlations between the QOLIBRI-OS-KID/ADO and its long version and the PedsQL, assessing generic HRQoL, as evidence of convergence. Correlations between the GAD-7, the PHQ-9, and the PCSI-SR8/13 were anticipated to be small to medium and negative, indicating some overlap between the constructs and suggesting that higher symptom burden is associated with lower HRQoL and vice versa, but measuring different constructs as an evidence of divergence. Determination coefficient (R2) was computed to evaluate the amount of explained variance.
Hypotheses were tested using one-tailed t-tests in the pTBI sample. We presumed that girls, adolescents (aged 13–17 years), participants with inferior recovery (i.e., KOSCHI < 5b), and participants with greater symptom burden (i.e., GAD-7 and PHQ-9 scores > 4, and PCSI-SR8/13 scores ≥ M + 1SD), would report lower HRQoL than the boys, children (aged 8–12 years), participants with superior recovery, and those reporting no symptom burden, respectively (see Table 1, column “Cut-off applied” for more details on the cut-offs for the test instruments). These assumptions, also tested for the QOLIBRI-KID/ADO, were based on findings from previous studies [13]. Cohen’s d was calculated to determine effect size, with values of 0.20, 0.50, and 0.80 representing small, medium, and large effects, respectively [39]. Results were reported only for groups with n > 30.
Prior to establishing reference values, we conducted a multi-group confirmatory factor analyses (CFA) [40, 41] to compare HRQoL construct assessment between the pTBI and the general population sample and determine measurement invariance (MI). We estimated three models with increasing constraints and then compared them using the χ2 difference test. We first fitted the configural model, then assumed equality of thresholds, followed by equality of both thresholds and loadings. If the difference test yields a non-significant result (p ≥ 0.05), it suggests that the HRQoL construct is assessed equally in both groups and that the difference between samples is solely due to the HRQoL disparity. Thus, the QOLIBRI-OS-KID/ADO can be utilized in both general population and TBI samples, and providing reference values is appropriate. Furthermore, we estimated a multiple linear regression to identify significant interactions between gender, age, and presence of chronic health conditions and the QOLIBRI-OS-KID/ADO total score for possible stratification of the reference values (see Supplementary Material, Regression analyses).
Finally, the reference values were calculated using percentiles, allowing for a simple and straightforward interpretation of the patient’s scores in relation to a comparable general population without a history of pTBI. The distribution of QOLIBRI-OS-KID/ADO scores in the general population sample was divided into percentiles (i.e., 2.5%, 5%, 16%, 30%, 40%, 50%, 60%, 70%, 85%, 95%, and 97.5%), with values below the 16th percentile indicating below-average HRQoL [42] as compared to the general population and thus a clinically relevant impairment.
We used R version 4.3.0 [43] for all analyses, employing the packages Table 1 [44], psych [45], lordif [29], and lavaan [46]. Unless otherwise stated, the significance level was set at 5%.
Results
Participants
Overall, 297 children and adolescents (54.2% male; mean age 12.6 ± 2.67 years) after pTBI participated in the study. Most sustained a mild TBI (79.8%), with 7% having visible intracranial imaging abnormalities. Injury occurred on average 5.36 ± 2.70 years prior to study entry with falls being the most common cause (72.1%). Most participants had fully recovered (86.9%). The mean QOLIBRI-OS-KID/ADO score was 82.3 ± 13.7 points. A significant difference was observed when comparing HRQoL between participants surveyed in different settings (i.e., online vs. offline), with offline respondents (40.4% of the sample) being slightly more satisfied (ΔM = 3.19, d = 0.23, p = 0.049) than those interviewed online (59.6%). A subsample of n = 57 participants completed the QOLIBRI-OS-KID/ADO twice, with a mean test–retest interval of 12.12 ± 2.38 days.
Overall, 1997 children and adolescents (49.5% male; mean age 12.4 ± 2.85 years) from the German general population participated in the study. Most had no chronic health problems according to parental reports (87.5%). The mean QOLIBRI-OS-KID/ADO score was 77.6 ± 16.7 points. The HRQoL of the general population sample was significantly lower than that of the pTBI sample (ΔM = − 4.66, d = − 0.29, p < 0.001). When comparing the subgroup of participants from the general population sample without chronic health conditions to the pTBI sample, the effect decreased slightly but remained significant (ΔM = − 4.04, d = − 0.26, p < 0.001). For details, see Table 2.
Item characteristics, reliability, and DIF analyses
At the item level, participants in both samples tended to indicate a rather high level of HRQoL, as the mean scores per item were around 4, corresponding to the response category “Quite (satisfied)”. As a result, the items were moderately to highly skewed.
Reliability coefficients were α = 0.75 and ω = 0.81 (pTBI sample) and α = 0.85 and ω = 0.86 (general population sample). The exclusion of none of the items increased the initial Cronbach’s α of the scale. The CITCs were > 0.40 in both samples. The DIF analyses suggested no meaningful differences in the items between children and adolescents. Although two items (i.e., “Autonomy” and “Future Prospects”) exhibited significant p-values in the general population sample, McFadden’s R2 values were less than 0.01, suggesting that these differences were negligible. The QOLIBRI-OS-KID/ADO data from children and adolescents were considered acceptable for joint analyses. For details, see Table 3.
The test–retest reliability in the pTBI sample was ICC(2,1) = 0.68, CI95% [0.51, 0.80], with a SEm of 6.35 points. A change of MDC = 17.59 points in the total score was considered a “true” change in HRQoL.
Validity
The one-dimensional factorial structure showed a good fit in both samples, with most indices being within acceptable ranges. The scaled fit statistics for the pTBI sample from the model using the WLS estimator were χ2(9) = 20.01, p = 0.018, χ2/df = 2.22, CFI = 0.98, TLI = 0.97, RMSEA[90% CI] 0.06 [0.03, 0.10], and SRMR = 0.05. The robust fit measures additionally derived from the model using the MLR estimator were CFI = 0.96, TLI = 0.94, RMSEA[90% CI] 0.07 [0.03, 0.11], SRMR = 0.04. The scaled fit statistics for the general population sample were χ2(9) = 119.77, p < 0.001, χ2/df = 13.31, CFI = 0.99, TLI = 0.98, RMSEA[90% CI] 0.08 [0.07, 0.09], and SRMR = 0.03. Robust fit measures were CFI = 0.98, TLI = 0.97, RMSEA[90% CI] 0.07 [0.05, 0.09], SRMR = 0.03 Fig. 2 provides an overview on standardized model parameters for the models using the WLS estimator.
Convergent validity analyses showed that the correlation coefficients between the QOLIBRI-OS-KID/ADO and the QOLIBRI-KID/ADO ranged from 0.45 (Emotions scale) to 0.82 (Total score). The respective amount of variance explained varied from 20% to 67%. The association with the PedsQL measuring generic HRQoL ranged from 0.52 (Social Functioning) to 0.74 (Total score), with variance explained between 27% and 55%, respectively.
Divergent validity analyses results revealed hypothesized negative associations between the QOLIBRI-OS-KID/ADO total score and instruments measuring symptom burden (i.e., GAD-7, PHQ-9, and PCSI-SR8/SR13) in the pTBI sample. The coefficients ranged from -0.29 (anxiety) to -0.56 (post-concussion symptoms), explaining 8% to 31% of the variability of the scores. For details, see Table 4.
Adolescents (ΔM = − 5.26, d = − 0.39, p < 0.001), participants with parent-reported post-pTBI health problems (ΔM = − 5.21, d = − 0.39, p < 0.001), those with higher levels of anxiety (ΔM = − 6.88, d = − 0.51, p < 0.001), depression (ΔM = − 8.77, d = − 0.67, p < 0.001), and post-concussion symptoms (ΔM = − 15.55, d = − 1.30, p < 0.001) had significantly lower HRQoL compared to the corresponding reference groups. All other comparisons were not significant or did not reach a sufficient number of individuals in at least one group (Table 5).
Measurement invariance
The results of the MI analyses showed no significant differences between the models with increasing restrictions (p > 0.05), suggesting that the construct of HRQoL was measured equally in both samples. In view of these results, it seems appropriate to use the data obtained from the general population sample as a reference for the QOLIBRI-OS-KID/ADO. For details, see Table 6.
Reference values
Based on the results of the regression analyses, the reference values were further stratified by gender and age, but excluding those sustaining chronic health conditions due to insufficient number of cases (see Supplementary Material, Regression analyses and Table S2). Table 7 provides reference values for the QOLIBRI-OS-KID/ADO total score. The following example illustrates the application of the reference values. A 15-year-old female patient has a QOLIBRI-OS-KID/ADO total score of 60. This means that her score is between the 5% and 16% percentiles, and she is in the under-average clinically relevant HRQoL range. Accordingly, compared to girls aged 13–17 from the general population, her HRQoL is below average and there is an indication for further diagnostics. In this case, a longer version of the instrument could be used to identify HRQoL domains that are particularly affected (e.g., cognition or emotions). Depending on the results, further diagnostics, such as neuropsychological testing or screening for depression or anxiety, may be performed to gain more information about what may have negatively impacted the patient’s HRQoL. If the QOLIBRI-OS-KID/ADO total score is in the normal range, there is no indication for further diagnostics.
Another interpretation would be to determine a cut-off for the assessment of clinical relevance. In this case, QOLIBRI-OS-KID/ADO scores less than or equal to 62 indicate impaired HRQoL in females aged 13–17 years. The score of 89 from our example is above the cut-off of 62, indicating that the patient did not report clinically relevant impairment.
An interactive app for the reference values can be found online (https://reference-values.shinyapps.io/Tables_Reference_values/, tab QOLIBRI-OS-KID/ADO; last access on 05.07.2023).
Discussion
The aim of this study was to investigate the psychometric properties of the QOLIBRI-OS-KID/ADO, a short scale assessing disease-specific HRQoL after pTBI, and to provide reference values from a general population sample. Based on the results of the psychometric analyses, we recommend the use of the QOLIBRI-OS-KID/ADO, especially when a brief overview of TBI-specific HRQoL is needed. The established reference values serve as a basis for screening decisions (e.g., further administration of a longer version of the instrument to gain more detailed insight into impaired HRQoL domains or omission of further steps in the absence of an indication) and provide information on the degree of overall HRQoL impairment after pTBI. Some of the central findings are discussed below.
The reliability of the QOLIBRI-OS-KID/ADO was satisfactory, indicating that the items are internally consistent, and the test results are repeatable within a period of 20 days. A score change of approximately 18 points represents a true change in HRQoL between two measurement occasions. Future studies should further focus on the clinically meaningful change to evaluate the course of recovery and the effectiveness of therapies and treatments after pTBI.
The DIF analyses suggested that the instrument is applicable to children and adolescents, allowing for aggregated analyses, item-level comparison, and longitudinal HRQoL monitoring. The psychometric properties of the QOLIBRI-OS-KID/ADO are similar to those of the QOLIBRI-KID/ADO [13], and the correlation between the total scores indicates strong construct overlap. The overlap with generic HRQoL as measured by the PedsQL was slightly lower. Similar associations between generic and disease-specific HRQoL measures have also been found for other pediatric health conditions (e.g., allergies [47], renal disease [48], or epilepsy [49]). This finding indicates that the constructs are related—also in terms of convergent validity—but not completely identical, suggesting that generic HRQoL measures cannot serve as substitutes for disease-specific PROMs.
Negative associations between the QOLIBRI-OS-KID/ADO and the instruments measuring symptom burden suggest that higher burden is associated with reduced disease-specific HRQoL and vice versa, which is consistent with findings for the QOLIBRI-KID/ADO [13, 14]. Finally, the goodness of fit of the one-factor model indicates that the assessment of HRQoL occurs unidimensionally and that the use of the QOLIBRI-OS-KID/ADO total score is reasonable. The use of both WLS and ML estimators in the CFA analyses supports this finding.
The QOLIBRI-OS-KID/ADO covers the HRQoL domains specified in the 35-item form in a general way and may therefore be less able to detect all differences between predefined sociodemographic and clinical groups than the long version. This may explain why no differences in sex, time since injury, and TBI severity were observed in the known group analyses in the pTBI sample. However, significant differences between age groups and between children and adolescents with and without post-injury health problems indicate that, as hypothesized, adolescents and those reporting health problems report lower HRQoL than comparison groups. This finding suggests that the QOLIBRI-OS-KID/ADO reflects the ability of the 35-item version to discriminate between predefined groups [13]. In addition, significantly lower QOLIBRI-OS-KID/ADO scores were observed in children and adolescents with higher levels of symptom burden compared to the low symptom burden groups. This reflects the findings from the previous studies suggesting decreased overall HRQoL in children with post-pTBI affective disorders [50] and post-concussion symptoms in both the acute [51] and post-acute phases [52]. Similar to the results of the long version [14], we did not observe significant differences in HRQoL between mild and moderate-to-severe pTBI groups. This finding may be explained by the small number of children and adolescents with greater TBI severity, which also reflects the prevalence of more severe TBI in pediatric populations [53], and thus lower power to detect the differences. Given that the adult version of the instrument showed a clear ability to discriminate between different groups of TBI severity at three, six, and 12 months after injury in a large pan-European study [17], further evidence is warranted to support the utility of the QOLIBRI-OS-KID/ADO across all severity levels in children and adolescents.
We found significantly lower HRQoL in the general population sample compared to the pTBI sample, although the effect was small. This may seem surprising at first, but this finding can be further explained. First, the pTBI sample included children and adolescents in the post-acute phase after mild injury. Therefore, the children and adolescents may have mostly recovered from the injury and/or adapted to its consequences, also in terms of HRQoL [54]. Second, by removing the reference to a specific health condition, as was done in adapting the QOLIBRI-OS-KID/ADO for administration to the general population, a broader or different interpretation of life and health domains to which responses can be related may be possible, resulting in greater variability in responses. Finally, due to the large size of the general population sample, the test may have been overpowered, detecting small effects that may be less relevant from a content-related perspective. In fact, the average difference between the samples was 4 points on a scale of 0–100. Future analyses using a matched sample adjusted for age, sex, and presence of chronic health conditions may shed more light on these differences.
Irrespective of gender and age, a cut-off of 62 on the QOLIBRI-OS-KID/ADO total score can be considered clinically relevant. For boys aged 8–12 years and 13–17 years, scores below 66 and 67, respectively, indicate impaired HRQoL, and for girls in both age groups, 67 and 62, respectively. With these scores in mind, possible deficits can be identified, and more detailed and personalized diagnostics can be applied to better understand which HRQoL domains are affected and need therapeutic interventions. Compared to the previously established cut-off value of 52 for the adult version of the questionnaire [55], the critical HRQoL score in children and adolescents is higher. However, the adult cut-off value was methodologically derived from the norm-based Mental Component Summary score of the Short Form Survey [56] using regression analyses and not from the comparable general adult population sample.
Strengths and limitations
The main strength of this study is that it provides a first short scale assessing disease-specific HRQoL after pTBI in the German-speaking language context, complemented by reference values obtained from a German general population. The study fills the gap of economic PROMs and provides interpretation guidelines in the field of pTBI. However, some limitations should be mentioned.
Overall, the number of children and adolescents after pTBI enrolled in the study represents 9% of the total number of invitations sent. Participation in the study was voluntary, and the decision to enroll should have been made by the parents. For privacy reasons, we were unable to perform analyses on known characteristics between those who enrolled and those who did not. Therefore, we can only assume that there may have been several reasons for non-response (ranging from change of address due to relocation, unwillingness to participate, or concerns about re-traumatizing the children through repeated explicit confrontation with the traumatic event). However, the planned number of participants in the project has been reached [14]. These issues have been addressed in more detail elsewhere [14].
The children and adolescents included in the present study mostly suffered from mild pTBI (ca. 80%) and were in the post-acute stage of injury (5.36 ± 2.70 years). Therefore, further validation in a more acute sample and in moderate to severe pTBI is warranted. The same applies to the levels of recovery, which could not be compared due to the small number of cases in the less favorable recovery groups: good recovery (KOSCHI 5a: 7.1%) and upper moderate disability (KOSCHI 4b: 5.1%). Further research is needed to better understand the applicability of the QOLIBRI-OS-KID/ADO total score to differentiate between different levels of recovery/disability.
Furthermore, the significant difference in HRQoL between online and offline interviewed patients can be explained by the higher prevalence of children and adolescents with pTBI within the first year (8.4% vs. 0%) and of moderate and severe TBI (23.4% vs. 14.8%) in the online group.
The general population sample was recruited online, which has both advantages and disadvantages. The main advantage is the accessibility of the relatively large number of participants. The potential disadvantages are a certain bias (e.g., recruitment of individuals with Internet access), non-standardized setting of data collection, and difficulty in verifying individuals [57]. Nevertheless, we have tried to maintain data quality by recruiting participants from internationally recognized market research agencies, controlling for inconsistent responses, and excluding “speeders” from the data set. While the planned number of cases of N = 2000 was almost reached also after data cleaning, the number of survey starts that ended with a termination was 61% (response rate 39%), indicating a possible selection bias and that only those interested completed the questionnaires. However, comparative analyses between respondents and non-respondents were not possible because the research agencies were not allowed to disclose information about those who had withdrawn.
The subsample with chronic health conditions was not sufficient to stratify the reference values. Therefore, the reference values provided represent the ideal norm. Expanding the reference values with information from subgroups with chronic health conditions would provide additional insight into HRQoL in the general population and allow for more nuanced comparisons with pTBI patients.
Conclusions
The QOLIBRI-OS-KID/ADO is a short, reliable, and valid PROM assessing disease-specific HRQoL after pTBI. Combined with reference values obtained from a general population sample, it provides a cost-effective screening for clinical practice and research. Future studies should focus on its applicability in more acute and severe samples and on the long-term validity, especially in combination with the adult version, which would provide further insight into the longitudinal effects of pTBI. Finally, translation into other languages would allow multinational studies to fill the knowledge gap regarding pTBI-specific HRQoL internationally.
Data availability
The data presented in this study are available on reasonable request from the corresponding author. Data are not publicly available for privacy reasons.
Abbreviations
- CFA:
-
Confirmatory factory analysis
- CFI:
-
Comparative fit index
- CI:
-
Confidence interval
- CITC:
-
Corrected item-total correlations
- DIF:
-
Differential item functioning
- GAD-7:
-
Generalized anxiety disorder 7
- HRQoL:
-
Health-related quality of life
- LORDIF:
-
Logistic ordinal regression differential item functioning
- MDC:
-
Minimal detectable change
- MI:
-
Measurement invariance
- PCSI-SR:
-
Postconcussion inventory—self report
- PedsQL:
-
Pediatric quality of life inventory
- PHQ-9:
-
Patient health questionnaire 9
- PROM:
-
Patient-reported outcome measure
- pTBI:
-
Pediatric traumatic brain injury
- QOLIBRI-KID/ADO:
-
Quality of life after brain injury for children and adolescents
- OLIBRI-OS-KID/ADO:
-
Quality of life after brain injury—overall scale for children and adolescents
- RAVLT:
-
Rey auditory verbal learning test
- RMSEA:
-
Root mean square error of approximation
- SEm:
-
Standard error of measurement
- SRMR:
-
Standardized root mean square residual
- TBI:
-
Traumatic brain injury
- TLI:
-
Tucker-Lewis index
References
Araki, T., Yokota, H., & Morita, A. (2017). Pediatric traumatic brain injury: Characteristic features, diagnosis, and management. Neurologia Medico-Chirurgica (Tokyo), 57, 82–93. https://doi.org/10.2176/nmc.ra.2016-0191
Babikian, T., Merkley, T., Savage, R. C., et al. (2015). Chronic aspects of pediatric traumatic brain injury: Review of the literature. Journal of Neurotrauma, 32, 1849–1860. https://doi.org/10.1089/neu.2015.3971
Stancin, T., Drotar, D., Taylor, H. G., et al. (2002). Health-related quality of life of children and adolescents after traumatic brain injury. Pediatrics, 109, E34. https://doi.org/10.1542/peds.109.2.e34
Wilde, E. A., Whiteneck, G. G., Bogner, J., et al. (2010). Recommendations for the use of common outcome measures in traumatic brain injury research. Archives of Physical Medicine and Rehabilitation, 91, 1650–1660.e17. https://doi.org/10.1016/j.apmr.2010.06.033
Wiebe, S., Guyatt, G., Weaver, B., et al. (2003). Comparative responsiveness of generic and specific quality-of-life instruments. Journal of Clinical Epidemiology, 56, 52–60. https://doi.org/10.1016/s0895-4356(02)00537-1
von Steinbuechel, N., Covic, A., Polinder, S., et al. (2016). Assessment of health-related quality of life after TBI: Comparison of a disease-specific (QOLIBRI) with a generic (SF-36) instrument. Behavioural Neurology, 2016, 1–14. https://doi.org/10.1155/2016/7928014
Varni, J. W., Seid, M., & Rode, C. A. (1999). The PedsQL: Measurement model for the pediatric quality of life inventory. Medical Care, 37, 126–139. https://doi.org/10.1097/00005650-199902000-00003
Cohen, M. L., Tulsky, D. S., Boulton, A. J., et al. (2019). Reliability and construct validity of the TBI-QOL communication short form as a parent-proxy report instrument for children with traumatic brain injury. Journal of Speech, Language, and Hearing Research, 62, 84–92. https://doi.org/10.1044/2018_JSLHR-L-18-0074
Varni, J. W., Limbers, C. A., & Burwinkle, T. M. (2007). How young can children reliably and validly self-report their health-related quality of life?: An analysis of 8,591 children across age subgroups with the PedsQLTM 4.0 generic core scales. Health and Quality of Life Outcomes, 5, 1. https://doi.org/10.1186/1477-7525-5-1
Upton, P., Lawford, J., & Eiser, C. (2008). Parent–child agreement across child health-related quality of life instruments: A review of the literature. Quality of Life Research, 17, 895–913. https://doi.org/10.1007/s11136-008-9350-5
Pieper, P., & Garvan, C. (2015). Concordance of child and parent reports of health-related quality of life in children with mild traumatic brain or non-brain injuries and in uninjured children: longitudinal evaluation. Journal of Pediatric Health Care, 29, 343–351. https://doi.org/10.1016/j.pedhc.2015.01.008
Hwang, H.-F., Chen, C.-Y., & Lin, M.-R. (2017). Patient-proxy agreement on the health-related quality of life one year after traumatic brain injury. Archives of Physical Medicine and Rehabilitation, 98, 2540–2547. https://doi.org/10.1016/j.apmr.2017.05.013
von Steinbuechel, N., Zeldovich, M., Greving, S., et al. (2023). Quality of life after brain injury in children and adolescents (QOLIBRI-KID/ADO)—The first disease-specific self-report questionnaire after traumatic brain injury. JCM, 12, 4898. https://doi.org/10.3390/jcm12154898
von Steinbuechel, N., Zeldovich, M., Timmermann, D., et al. (2024). Final validation of the quality of life after brain injury for children and adolescents (QOLIBRI-KID/ADO) questionnaire. Children. https://doi.org/10.3390/children11040438
von Steinbuechel, N., Wilson, L., Gibbons, H., et al. (2010). Quality of life after brain injury (QOLIBRI): Scale development and metric properties. Journal of Neurotrauma, 27, 1167–1185. https://doi.org/10.1089/neu.2009.1076
von Steinbuechel, N., Wilson, L., Gibbons, H., et al. (2012). QOLIBRI overall scale: A brief index of health-related quality of life after traumatic brain injury. Journal of Neurology, Neurosurgery and Psychiatry, 83, 1041–1047. https://doi.org/10.1136/jnnp-2012-302361
von Steinbuechel, N., Rauen, K., Covic, A., et al. (2023). Sensitivity of outcome instruments in a priori selected patient groups after traumatic brain injury: Results from the CENTER-TBI study. PLoS ONE, 18, e0280796. https://doi.org/10.1371/journal.pone.0280796
Teasdale, G., & Jennett, B. (1974). Assessment of coma and impaired consciousness: A practical scale. Lancet, 2, 81–84. https://doi.org/10.1016/s0140-6736(74)91639-0
Mundfrom, D. J., Shaw, D. G., & Ke, T. L. (2005). Minimum sample size recommendations for conducting factor analyses. International Journal of Testing, 5, 159–168. https://doi.org/10.1207/s15327574ijt0502_4
Spitzer, R. L., Kroenke, K., Williams, J. B. W., & Löwe, B. (2006). A brief measure for assessing generalized anxiety disorder: The GAD-7. Archives of Internal Medicine, 166, 1092–1097. https://doi.org/10.1001/archinte.166.10.1092
Sequeira, S. L., Morrow, K. E., Silk, J. S., et al. (2021). National norms and correlates of the PHQ-8 and GAD-7 in parents of school-age children. Journal of Child and Family Studies, 30, 2303–2314. https://doi.org/10.1007/s10826-021-02026-x
Kroenke, K., & Spitzer, R. L. (2002). The PHQ-9: A new depression diagnostic and severity measure. Psychiatric Annals, 32, 509–515. https://doi.org/10.3928/0048-5713-20020901-06
Richardson, L. P., McCauley, E., Grossman, D. C., et al. (2010). Evaluation of the patient health questionnaire (PHQ-9) for detecting major depression among adolescents. Pediatrics, 126, 1117–1123. https://doi.org/10.1542/peds.2010-0852
Sady, M. D., Vaughan, C. G., & Gioia, G. A. (2014). Psychometric characteristics of the postconcussion symptom inventory in children and adolescents. Archives of Clinical Neuropsychology, 29, 348–363. https://doi.org/10.1093/arclin/acu014
Müller, H., Hasse-Sander, I., Horn, R., et al. (1997). Rey auditory-verbal learning test: Structure of a modified German version. Journal of Clinical Psychology, 53, 663–671.
Strauss, E., Sherman, E. M., & Spreen, O. (2006). Rey auditory verbal learning test (RAVLT). A compendium of neuropsychological tests—Administration, norms, and commentary (pp. 776–810). Oxford University Press.
Calvert, S., Miller, H. E., Curran, A., et al. (2008). The king’s outcome scale for childhood head injury and injury severity and outcome measures in children with traumatic brain injury. Developmental Medicine & Child Neurology, 50, 426–431. https://doi.org/10.1111/j.1469-8749.2008.02061.x
Bulmer, M. G. (1979). Principles of statistics. Dover Publications.
Choi, S., Gibbons, L., & Crane, P. (2011). Lordif: An R package for detecting differential item functioning using iterative hybrid ordinal logistic regression/item response theory and Monte Carlo simulations. Journal of Statistical Software, 39, 1–30. https://doi.org/10.18637/jss.v039.i08
Terwee, C. B., Bot, S. D. M., de Boer, M. R., et al. (2007). Quality criteria were proposed for measurement properties of health status questionnaires. Journal of Clinical Epidemiology, 60, 34–42. https://doi.org/10.1016/j.jclinepi.2006.03.012
Zinbarg, R. E., Revelle, W., Yovel, I., & Li, W. (2005). Cronbach’s α, Revelle’s β, and Mcdonald’s ωH: Their relations with each other and two alternative conceptualizations of reliability. Psychometrika, 70, 123–133. https://doi.org/10.1007/s11336-003-0974-7
The Whoqol Group. (1998). The World Health Organization quality of life assessment (WHOQOL): Development and general psychometric properties. Social Science & Medicine, 46, 1569–1585. https://doi.org/10.1016/S0277-9536(98)00009-4
Janssens, L., Gorter, J. W., Ketelaar, M., et al. (2008). Health-related quality-of-life measures for long-term follow-up in children after major trauma. Quality of Life Research, 17, 701–713.
Harvill, L. M. (1991). An NCME instructional module on: Standard error of measurement. Educational Measure: Issues Practice, 10, 33–41. https://doi.org/10.1111/j.1745-3992.1991.tb00195.x
Cole, D. A. (1987). Utility of confirmatory factor analysis in test validation research. Journal of Consulting and Clinical Psychology, 55, 584–594. https://doi.org/10.1037/0022-006X.55.4.584
Hu, L., & Bentler, P. M. (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6, 1–55. https://doi.org/10.1080/10705519909540118
Steiger, J. H. (2016). Notes on the Steiger-Lind (1980) Handout. Structural Equation Modeling: A Multidisciplinary Journal, 23, 777–781. https://doi.org/10.1080/10705511.2016.1217487
Xia, Y., & Yang, Y. (2019). RMSEA, CFI, and TLI in structural equation modeling with ordered categorical data: The story they tell depends on the estimation methods. Behavior Research Methods, 51, 409–428. https://doi.org/10.3758/s13428-018-1055-2
Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale: L. Erlbaum Associates.
Wu, H., & Estabrook, R. (2016). Identification of confirmatory factor analysis models of different levels of invariance for ordered categorical outcomes. Psychometrika, 81, 1014–1045. https://doi.org/10.1007/s11336-016-9506-0
Svetina, D., Rutkowski, L., & Rutkowski, D. (2020). Multiple-group invariance with categorical outcomes using updated guidelines: An illustration using mplus and the lavaan/semtools packages. Structural Equation Modeling: A Multidisciplinary Journal, 27, 111–130. https://doi.org/10.1080/10705511.2019.1602776
Ware, J. E., Kosinski, M., Bjorner, J. B., et al. (2007). User’s manual for the SF-36v2 health survey (2nd ed.). Lincoln, RI.
R Core Team. (2023). R: A language and environment for statistical computing. R Core Team.
Rich B (2021) Table1: Tables of descriptive statistics in HTML
Revelle, W. (2021). An introduction to the psych package: Part II scale construction and psychometrics. Northwestern University.
Rosseel, Y. (2012). Lavaan: An R package for structural equation modeling. Journal of Statistical Software. https://doi.org/10.18637/jss.v048.i02
Kiotseridis, H., Cilio, C. M., Bjermer, L., et al. (2013). Quality of life in children and adolescents with respiratory allergy, assessed with a generic and disease-specific instrument. The Clinical Respiratory Journal, 7, 168–175. https://doi.org/10.1111/j.1752-699X.2012.00298.x
Goldstein, S. L., Graham, N., Warady, B. A., et al. (2008). Measuring health-related quality of life in children with ESRD: Performance of the generic and ESRD-specific instrument of the pediatric quality of life inventory (PedsQL). American Journal of Kidney Diseases, 51, 285–297. https://doi.org/10.1053/j.ajkd.2007.09.021
Baca, C. B., Vickrey, B. G., Vassar, S., & Berg, A. T. (2015). Disease-targeted versus generic measurement of health-related quality of life in epilepsy. Quality of Life Research, 24, 1379–1387. https://doi.org/10.1007/s11136-014-0867-5
Martin-Herz, S. P., Zatzick, D. F., & McMahon, R. J. (2012). Health-related quality of life in children and adolescents following traumatic injury: A review. Clinical Child and Family Psychology Review, 15, 192–214. https://doi.org/10.1007/s10567-012-0115-x
Novak, Z., Aglipay, M., Barrowman, N., et al. (2016). Association of persistent postconcussion symptoms with pediatric quality of life. JAMA Pediatrics, 170, e162900. https://doi.org/10.1001/jamapediatrics.2016.2900
Fineblit, S., Selci, E., Loewen, H., et al. (2016). Health-related quality of life after pediatric mild traumatic brain injury/concussion: A systematic review. Journal of Neurotrauma, 33, 1561–1568. https://doi.org/10.1089/neu.2015.4292
Dewan, M. C., Mummareddy, N., Wellons, J. C., & Bonfield, C. M. (2016). Epidemiology of global pediatric traumatic brain injury: Qualitative review. World Neurosurgery, 91, 497–509.e1. https://doi.org/10.1016/j.wneu.2016.03.045
Gould, K. R., & Ponsford, J. L. (2015). A longitudinal examination of positive changes in quality-of-life after traumatic brain injury. Brain Injury, 29, 283–290. https://doi.org/10.3109/02699052.2014.974671
Wilson, L., Marsden-Loftus, I., Koskinen, S., et al. (2017). Interpreting quality of life after brain injury scores: Cross-walk with the short form-36. Journal of Neurotrauma, 34, 59–65. https://doi.org/10.1089/neu.2015.4287
Ware, J. E., & Sherbourne, C. D. (1992). The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Medical Care, 30, 473–483.
Ball, H. L. (2019). Conducting online surveys. Journal of Human Lactation, 35, 413–417. https://doi.org/10.1177/0890334419848734
Acknowledgements
The authors are grateful to all study participants and their families for their contributions to pediatric TBI research. The authors would like to acknowledge all the participants and investigators, as well as all the experts who supported the research project.
In particular, we would like to thank all of the investigators who have made it possible to collect data from children and adolescents after TBI used in the comparative analyses: Mattea Ausmeier, Agnes Berghuber, Rieke Boeddeker, Hanna Boenitz, Elena Bonke, Lea Busch, Helena Duewel, Meike Engelbrecht, Nicole Fabri, Anastasia Gorbunova, Shaghayegh Gorji, Louisa Harmsen, Korbinian Heinrich, Sophia Hierlmayer, Stefan Hillmann, Leonard B.Jung, Alexander Kaiser, Sina Kantelhardt, Hanna Klaeger, Maximilian Kluge, Celine Koenig, Ugne Krenz, Lena Kuschel, Clara Lamersdorff, Louisa Lohrberg, Katja Lorenz, Johann de Maeyer, Isabelle Mueller, Philine Mueller, Sophia Mueller, Benjamin Nast-Kolb, Carl Jannes Neuse, Lara Pankatz, Johanna von Petersdorff, Jonas Pietersteiner, Julius Poppel, Paul S. Raffelhüschen, Anna-Lena Raidl, Nico Rodo, Maren Roehl, Philine Rojczyk, Dorle Schaper, Emma Schmiedekind, Nils Schoenberg, Paula von Schorlemer, Carolin Singelmann, Victoria Stefan, Inga Steppacher, Dagmar Timmermann, Lisa F. Umminger, and Tim L. T. Wiegand. We would also like to thank the additional national and international experts supporting us for the duration of the project, especially during the item generation and reduction process. National experts: Claudia Armgardt, Monika Bullinger, Katharina Diepold, Christiane Gruß, Ralf Heindorf, Dirk Heinicke, Rainer John, Dorothea Mielke, Ulrike Neirich, Bettina Schulz, Gertrud Wietholt, Angela Woller, and Joy Weber. International experts: Anna Adlam, Nada Andelic, Juan Carlos Arango-Lasprilla, Miriam Beauchamp, Jeroen Bekkers, Emily Bennett, Enrico Castelli, Mathilde Chevignard, Gemma Costello, Rita Formisano, Rob Forsyth, Carol Hawley, Fiona Lecky, Marianne Løvstad, Mari Saarinen, Shannon Scratch, Suzanna Watson, and Shari Wade.
Funding
Open access funding provided by University of Innsbruck and Medical University of Innsbruck. This work was supported by Dr. Senckenbergische Stiftung/Clementine Kinderhospital Dr. Christ ‘sche Stiftungen (Germany), Deutsche Gesetzliche Unfallversicherung (Germany; grant number FR282), and Uniscientia Stiftung (Switzerland).
Author information
Authors and Affiliations
Contributions
Marina Zeldovich and Nicole von Steinbuechel contributed to the study conception and design. Data collection was performed by Katrin Cunitz, Inga K. Koerte, Matthias Kieslich, Marlene Henrich, Knut Brockmann, Michael Lendt, Christian Auer, Axel Neu, Joenna Driemeyer, Ulrike Wartemann, Claudius Thomé, Daniel Pinggera, Steffen Berweck, Michaela Bonfert, Joachim Suss (pTBI sample) as well as supervised by Marina Zeldovich (general population sample). Data analyses were performed by Marina Zeldovich and Leonie Krol. The first draft of the manuscript was written by Marina Zeldovich and Leonie Krol and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Both studies were conducted in accordance with all relevant local laws and regulations, including but not limited to the ICH Harmonized Tripartite Guideline for Good Clinical Practice (“ICH GCP”) and the World Medical Association’s Declaration of Helsinki (“Ethical Principles for Medical Research Involving Human Subjects”). The Ethics Committee of the University Medical Center Göttingen has approved the studies (Application Number 19/4/18).
Consent to participate
Written informed consent was obtained from the parents or legal guardians.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zeldovich, M., Krol, L., Koerte, I.K. et al. A short scale to measure health-related quality of life after traumatic brain injury in children and adolescents (QOLIBRI-OS-KID/ADO): psychometric properties and German reference values. Qual Life Res (2024). https://doi.org/10.1007/s11136-024-03764-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s11136-024-03764-3