Abstract
Purpose
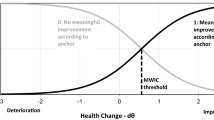
The minimal important change (MIC) is defined as the smallest within-individual change in a patient-reported outcome measure (PROM) that patients on average perceive as important. We describe a method to estimate this value based on longitudinal confirmatory factor analysis (LCFA). The method is evaluated and compared with a recently published method based on longitudinal item response theory (LIRT) in simulated and real data. We also examined the effect of sample size on bias and precision of the estimate.
Methods
We simulated 108 samples with various characteristics in which the true MIC was simulated as the mean of individual MICs, and estimated MICs based on LCFA and LIRT. Additionally, both MICs were estimated in existing PROMIS Pain Behavior data from 909 patients. In another set of 3888 simulated samples with sample sizes of 125, 250, 500, and 1000, we estimated LCFA-based MICs.
Results
The MIC was equally well recovered with the LCFA-method as using the LIRT-method, but the LCFA analyses were more than 50 times faster. In the Pain Behavior data (with higher scores indicating more pain behavior), an LCFA-based MIC for improvement was estimated to be 2.85 points (on a simple sum scale ranging 14–42), whereas the LIRT-based MIC was estimated to be 2.60. The sample size simulations showed that smaller sample sizes decreased the precision of the LCFA-based MIC and increased the risk of model non-convergence.
Conclusion
The MIC can accurately be estimated using LCFA, but sample sizes need to be preferably greater than 125.






Similar content being viewed by others

Data availability
The R-code used to simulate and analyze the samples is provided in the Supplementary material.
The empirical example data can be obtained from the first author on reasonable request.
Code availability
The R-code is provided in the Supplementary material.
References
FDA. (2019). Incorporating clinical outcome assessments into endpoints for regulatory decision-making. US Food and Drug Administration.
Jaeschke, R., Singer, J., & Guyatt, G. H. (1989). Measurement of health status. Ascertaining the minimal clinically important difference. Controlled Clinical Trials, 10, 407–415.
Terwee, C. B., Peipert, J. D., Chapman, R., Lai, J. S., Terluin, B., Cella, D., Griffith, P., & Mokkink, L. B. (2021). Minimal important change (MIC): A conceptual clarification and systematic review of MIC estimates of PROMIS measures. Quality of Life Research, 30(10), 2729–2754.
King, M. T. (2011). A point of minimal important difference (MID): A critique of terminology and methods. Expert Review of Pharmacoeconomics & Outcomes Research, 11, 171–184.
Terluin, B., Eekhout, I., & Terwee, C. B. (2017). The anchor-based minimal important change, based on receiver operating characteristic analysis or predictive modeling, may need to be adjusted for the proportion of improved patients. Journal of Clinical Epidemiology, 83, 90–100.
Vanier, A., Sebille, V., Blanchin, M., & Hardouin, J. B. (2021). The minimal perceived change: A formal model of the responder definition according to the patient’s meaning of change for patient-reported outcome data analysis and interpretation. BMC Medical Research Methodology, 21(1), 128.
Bjorner, J. B., Terluin, B., Trigg, A., Hu, J., Brady, K. J. S., & Griffiths, P. (2023). Establishing thresholds for meaningful within-individual change using longitudinal item response theory. Quality of Life Research, 32(5), 1267–1276.
Takane, Y., & Deleeuw, J. (1987). On the relationship between item response theory and factor-analysis of discretized variables. Psychometrika, 52(3), 393–408.
Wirth, R. J., & Edwards, M. C. (2007). Item factor analysis: Current approaches and future directions. Psychological Methods, 12(1), 58–79.
Embretson, S. E., & Reise, S. P. (2009). Item response theory for psychologists (2nd ed.). Lawrence Erlbaum.
Kamata, A., & Bauer, D. J. (2008). A note on the relation between factor analytic and item response theory. Structural Equation Modeling-a Multidisciplinary Journal, 15(1), 136–153.
Samejima, F. (1996). The graded response model. In W. J. van der Linden & R. Hambleton (Eds.), Handbook of modern item response theory (pp. 85–100). Springer.
Chalmers, R. P. (2012). mirt: A multidimensional item response theory package for the R environment. Journal of Statistical Software, 48(6), 1–29.
Rosseel, Y. (2012). lavaan: An R package for structural equation modeling. Journal of Statistical Software, 48, 2.
R Core Team. (2016). R: A language and environment for statistical computing. R Foundation for Statistical Computing.
Schuller, W., Terwee, C. B., Klausch, T., Roorda, L. D., Rohrich, D. C., Ostelo, R. W., Terluin, B., & de Vet, H. C. W. (2019). Psychometric properties of the Dutch-Flemish patient-reported outcomes measurement information system pain behavior item bank in patients with musculoskeletal complaints. The Journal of Pain, 20(11), 1328–1337.
Crins, M. H. P., Roorda, L. D., Smits, N., de Vet, H. C. W., Westhovens, R., Cella, D., Cook, K. F., Revicki, D., van Leeuwen, J., Boers, M., Dekker, J., & Terwee, C. B. (2016). Calibration of the Dutch-Flemish PROMIS pain behavior item bank in patients with chronic pain. European Journal of Pain, 20(2), 284–296.
Gasparini, A. (2018). rsimsum: Summarise results from Monte Carlo simulation studies. Journal of Open Source Software, 3(26), 739.
Terluin, B., Griffiths, P., Trigg, A., Terwee, C. B., & Bjorner, J. B. (2022). Present state bias in transition ratings was accurately estimated in simulated and real data. Journal of Clinical Epidemiology, 143, 128–136.
Morris, T. P., White, I. R., & Crowther, M. J. (2019). Using simulation studies to evaluate statistical methods. Statistics in Medicine, 38(11), 2074–2102.
Deyo, R. A., & Centor, R. M. (1986). Assessing the responsiveness of functional scales to clinical change: An analogy to diagnostic test performance. J Chron Dis, 39, 897–906.
Terluin, B., Eekhout, I., Terwee, C. B., & de Vet, H. C. W. (2015). Minimal important change (MIC) based on a predictive modeling approach was more precise than MIC based on ROC analysis. Journal of Clinical Epidemiology, 68, 1388–1396.
Terluin, B., Eekhout, I., & Terwee, C. B. (2022). Improved adjusted minimal important change took reliability of transition ratings into account. Journal of Clinical Epidemiology, 148, 48–53.
Hays, R. D., Brodsky, M., Johnston, M. F., Spritzer, K. L., & Hui, K. K. (2005). Evaluating the statistical significance of health-related quality-of-life change in individual patients. Evaluation and the Health Professions, 28(2), 160–171.
Trigg, A., Lenderking, W. R., & Boehnke, J. R. (2023). Introduction to the special section: “Methodologies and considerations for meaningful change.” Qual Life Res. https://doi.org/10.1007/s11136-023-03413-1
Terluin, B., Koopman, J. E., Hoogendam, L., Griffiths, P., Terwee, C. B., & Bjorner, J. B. (2023). Estimating meaningful thresholds for multi-item questionnaires using item response theory. Quality of Life Research, 32(6), 1819–1830.
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
Conceptualization: BT; Methodology: BT, AT, JBB; Formal analysis and investigation: BT; Writing—original draft preparation: BT; Writing—review and editing: BT, AT, PF, WS, CBT, JBB; Supervision: JBB.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
With respect to the Pain Behavior data, the Medical Ethical Committee of the VU Medical Center at Amsterdam (2013/20) approved the study and accepted a verbal-informed consent procedure.
Consent to participate
Verbal informed consent was obtained from all patients providing the Pain Behavior data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Terluin, B., Trigg, A., Fromy, P. et al. Estimating anchor-based minimal important change using longitudinal confirmatory factor analysis. Qual Life Res 33, 963–973 (2024). https://doi.org/10.1007/s11136-023-03577-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-023-03577-w