Abstract
Introduction
Routine Electronic Monitoring of Health-Related Quality of Life (HRQoL) (REMOQOL) in clinical care with real-time feedback to physicians could help to enhance patient-centered care. We evaluated the feasibility of REMOQOL in the French context in the QOLIBRY study. The primary objective was to assess the patients’ compliance with REMOQOL.
Methods
The QOLIBRY study was a single-center, prospective study conducted in the University Hospital of Besançon (France). Eligible patients were those treated with systemic therapies for breast, lung or colorectal cancer at any stage. Patients were invited to complete the EORTC QLQ-C30 questionnaire and cancer-site-specific modules before each visit on tablets and/or computers in the hospital or at home. During the consultation, physicians had real-time access to visual summaries of HRQoL scores. Compliance was assessed as adequate if at least 66% of HRQoL assessments were completed during the 4 months of follow-up.
Results
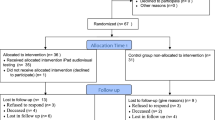
Between March 2016 and October 2018, 177 patients were included in the QOLIBRY study. Median age was 64 years (IQR 54–71). The proportion of patients with an adequate compliance rate was 95.5% (n = 63) in the breast cancer cohort, 98.2% (n = 55) in the colorectal cancer cohort, and 90.9% (n = 50) in the lung cancer cohort. The physicians checked the HRQoL results in 73.1% of visits and prescribed supportive care and adapted patient management in 8.3% and 5.2% of visits, respectively.
Conclusion & perspectives
The results of QOLIBRY study suggest that REMOQOL is feasible in the French context. However, information about HRQoL monitoring, training of the physicians in the use of the software, and recommendations for using HRQoL results to guide care are essential and must be improved.




Similar content being viewed by others
Abbreviations
- ANSM:
-
French national drug agency
- PRO:
-
Patient-reported outcome
- BC:
-
Breast cancer
- CHES:
-
Computer-based health evaluation system
- CRC:
-
Colorectal cancer
- EORTC:
-
European organization for research and treatment against cancer
- HRQoL:
-
Health-related quality of life
- LC:
-
Lung cancer
- REMOQOL:
-
Routine electronic monitoring of health-related quality of life
References
Bottomley, A., Pe, M., Sloan, J., et al. (2016). Analysing data from patient-reported outcome and quality of life endpoints for cancer clinical trials: A start in setting international standards. The Lancet Oncology, 17, e510–e514. https://doi.org/10.1016/S1470-2045(16)30510-1.
European Medicines Agency European Medicines Agency. (2016). Clinical efficacy and safety—Appendix 2 to the guideline on the evaluation of anticancer medicinal products in man—the use of patient-reported outcome (PRO) measures in oncology studies. Retrieved July 27, 2016 from http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_001127.jsp&mid=WC0b01ac0580034cf3.
Luckett, T., Butow, P. N., & King, M. T. (2009). Improving patient outcomes through the routine use of patient-reported data in cancer clinics: Future directions. Psychooncology, 18, 1129–1138. https://doi.org/10.1002/pon.1545.
Kotronoulas, G., Kearney, N., Maguire, R., et al. (2014). What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. JCO, 32, 1480–1501. https://doi.org/10.1200/JCO.2013.53.5948.
Howell, D., Molloy, S., Wilkinson, K., et al. (2015). Patient-reported outcomes in routine cancer clinical practice: A scoping review of use, impact on health outcomes, and implementation factors. The Annals of Oncology, 26, 1846–1858. https://doi.org/10.1093/annonc/mdv181.
King, S., Exley, J., Parks, S., et al. (2016). The use and impact of quality of life assessment tools in clinical care settings for cancer patients, with a particular emphasis on brain cancer: Insights from a systematic review and stakeholder consultations. Quality of Life Research, 25, 2245–2256. https://doi.org/10.1007/s11136-016-1278-6.
Yang, L. Y., Manhas, D. S., Howard, A. F., & Olson, R. A. (2018). Patient-reported outcome use in oncology: A systematic review of the impact on patient-clinician communication. Supportive Care in Cancer, 26, 41–60. https://doi.org/10.1007/s00520-017-3865-7.
Anatchkova, M., Donelson, S. M., Skalicky, A. M., et al. (2018). Exploring the implementation of patient-reported outcome measures in cancer care: Need for more real-world evidence results in the peer reviewed literature. The Journal of Patient-Reported Outcomes, 2, 64. https://doi.org/10.1186/s41687-018-0091-0.
Berry, D. L., Hong, F., Halpenny, B., et al. (2014). Electronic self-report assessment for cancer and self-care support: Results of a multicenter randomized trial. The Journal of Clinical Oncology, 32, 199–205. https://doi.org/10.1200/JCO.2013.48.6662.
Chen, J., Ou, L., & Hollis, S. J. (2013). A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Services Research, 13, 211. https://doi.org/10.1186/1472-6963-13-211.
Basch, E., Deal, A. M., Kris, M. G., et al. (2016). Symptom monitoring with patient-reported outcomes during routine cancer treatment: A randomized controlled trial. The Journal of Clinical Oncology, 34, 557–565. https://doi.org/10.1200/JCO.2015.63.0830.
Denis, F., Lethrosne, C., Pourel, N., et al. (2017). Randomized trial comparing a web-mediated follow-up with routine surveillance in lung cancer patients. The Journal of the National Cancer Institute. https://doi.org/10.1093/jnci/djx029.
Basch, E., Deal, A. M., Dueck, A. C., et al. (2017). Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA, 318, 197–198. https://doi.org/10.1001/jama.2017.7156.
Denis, F., Basch, E., Septans, A.-L., et al. (2019). Two-year survival comparing web-based symptom monitoring vs routine surveillance following treatment for lung cancer. JAMA, 321, 306–307. https://doi.org/10.1001/jama.2018.18085.
Detmar, S. B., Muller, M. J., Wever, L. D., et al. (2001). The patient-physician relationship. Patient-physician communication during outpatient palliative treatment visits: An observational study. JAMA, 285, 1351–1357.
Detmar, S. B., Muller, M. J., Schornagel, J. H., et al. (2002). Health-related quality-of-life assessments and patient-physician communication: A randomized controlled trial. JAMA, 288, 3027–3034.
Velikova, G., Booth, L., Smith, A. B., et al. (2004). Measuring quality of life in routine oncology practice improves communication and patient well-being: A randomized controlled trial. The Journal of Clinical Oncology, 22, 714–724. https://doi.org/10.1200/JCO.2004.06.078.
Wintner, L. M., Sztankay, M., Aaronson, N., et al. (2016). The use of EORTC measures in daily clinical practice-A synopsis of a newly developed manual. The European Journal of Cancer, 68, 73–81. https://doi.org/10.1016/j.ejca.2016.08.024.
Basch, E., & Snyder, C. (2017). Overcoming barriers to integrating patient-reported outcomes in clinical practice and electronic health records. The Annals of Oncology, 28, 2332–2333. https://doi.org/10.1093/annonc/mdx506.
Chan, E. K. H., Edwards, T. C., Haywood, K., et al. (2018). Implementing patient-reported outcome measures in clinical practice: A companion guide to the ISOQOL user’s guide. Quality of Life Research. https://doi.org/10.1007/s11136-018-2048-4.
Mouillet, G., Fritzsch, J., Paget-Bailly, S., et al. (2019). Health-related quality of life assessment for patients with advanced or metastatic renal cell carcinoma treated with a tyrosine kinase inhibitor using electronic patient-reported outcomes in daily clinical practice (QUANARIE trial): Study protocol. Health and Quality of Life Outcomes, 17, 1–9. https://doi.org/10.1186/s12955-019-1085-1.
Aaronson, N. K., Ahmedzai, S., Bergman, B., et al. (1993). The European organization for research and treatment of cancer QLQ-C30: A quality-of-life instrument for use in International Clinical Trials in Oncology. JNCI Journal of the National Cancer Institute, 85, 365–376. https://doi.org/10.1093/jnci/85.5.365.
Bergman, B., Aaronson, N. K., Ahmedzai, S., et al. (1994). The EORTC QLQ-LC13: A modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. The European Journal of Cancer, 30A, 635–642.
Sprangers, M. A., Groenvold, M., Arraras, J. I., et al. (1996). The European Organization for research and treatment of cancer breast cancer-specific quality-of-life questionnaire module: First results from a three-country field study. The Journal of Clinical Oncology, 14, 2756–2768. https://doi.org/10.1200/JCO.1996.14.10.2756.
Whistance, R. N., Conroy, T., Chie, W., et al. (2009). Clinical and psychometric validation of the EORTC QLQ-CR29 questionnaire module to assess health-related quality of life in patients with colorectal cancer. The European Journal of Cancer, 45, 3017–3026. https://doi.org/10.1016/j.ejca.2009.08.014.
Rabin, R., & de Charro, F. (2001). EQ-5D: A measure of health status from the EuroQol Group. Annals of Medicine, 33, 337–343. https://doi.org/10.3109/07853890109002087.
Holzner, B., Giesinger, J. M., Pinggera, J., et al. (2012). The Computer-based Health Evaluation Software (CHES): A software for electronic patient-reported outcome monitoring. BMC Medical Informatics and Decision Making, 12, 126. https://doi.org/10.1186/1472-6947-12-126.
Mouillet, G., Fritzsch, J., Thiery-Vuillemin, A., et al. (2019). Physicians’ satisfaction with health-related quality of life (HRQoL) assessment in daily clinical practice using electronic patient-reported outcome (ePRO) for cancer patients. Annals of Oncology, 30, v729–v730. https://doi.org/10.1093/annonc/mdz265.035.
Julious, S. A. (2005). Sample size of 12 per group rule of thumb for a pilot study. Pharmaceutical Statistics, 4, 287–291. https://doi.org/10.1002/pst.185.
Sim, J., & Lewis, M. (2012). The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. The Journal of Clinical Epidemiology, 65, 301–308. https://doi.org/10.1016/j.jclinepi.2011.07.011.
Fayers, P. M., Aaronson, N. K., Bjordal, K., et al. (1999). EORTC QLQ-C30 scoring manual. Brussels: Eortc.
R: The R Project for Statistical Computing (2020). Retrieved November 10, 2020 from https://www.r-project.org/index.html.
Schuler, M. K., Trautmann, F., Radloff, M., et al. (2016). Implementation of a mobile inpatient quality of life (QoL) assessment for oncology nursing. Supportive Care in Cancer, 24, 3391–3399. https://doi.org/10.1007/s00520-016-3163-9.
Bottomley, A., Vachalec, S., Bjordal, K., et al. (2002). The development and utilisation of the European Organisation for research and treatment of cancer quality of life group item bank. The European Journal of Cancer, 38, 1611–1614. https://doi.org/10.1016/s0959-8049(02)00125-9.
Snyder, C. F., Jensen, R. E., Geller, G., et al. (2010). Relevant content for a patient-reported outcomes questionnaire for use in oncology clinical practice: Putting doctors and patients on the same page. Quality of Life Research, 19, 1045–1055. https://doi.org/10.1007/s11136-010-9655-z.
Trautmann, F., Hentschel, L., Hornemann, B., et al. (2016). Electronic real-time assessment of patient-reported outcomes in routine care-first findings and experiences from the implementation in a comprehensive cancer center. Supportive Care in Cancer, 24, 3047–3056. https://doi.org/10.1007/s00520-016-3127-0.
Duncan, E. A. S., & Murray, J. (2012). The barriers and facilitators to routine outcome measurement by allied health professionals in practice: A systematic review. BMC Health Services Research, 12, 96. https://doi.org/10.1186/1472-6963-12-96.
Antunes, B., Harding, R., Higginson, I. J., & EUROIMPACT. (2014). Implementing patient-reported outcome measures in palliative care clinical practice: A systematic review of facilitators and barriers. Palliative Medicine, 28, 158–175. https://doi.org/10.1177/0269216313491619.
Mitchell, A. J. (2013). Screening for cancer-related distress: When is implementation successful and when is it unsuccessful? Acta Oncologica, 52, 216–224. https://doi.org/10.3109/0284186X.2012.745949.
Mejdahl, C. T., Schougaard, L. M. V., Hjollund, N. H., et al. (2020). Patient-reported outcome measures in the interaction between patient and clinician—A multi-perspective qualitative study. The Journal of Patient-Reported Outcomes, 4, 3. https://doi.org/10.1186/s41687-019-0170-x.
Velikova, G., Keding, A., Harley, C., et al. (2010). Patients report improvements in continuity of care when quality of life assessments are used routinely in oncology practice: Secondary outcomes of a randomised controlled trial. The European Journal of Cancer, 46, 2381–2388. https://doi.org/10.1016/j.ejca.2010.04.030.
Whittle, A. K., Kalsi, T., Babic-Illman, G., et al. (2017). A comprehensive geriatric assessment screening questionnaire (CGA-GOLD) for older people undergoing treatment for cancer. The European Journal of Cancer Care (Engl). https://doi.org/10.1111/ecc.12509.
Giesinger, J. M., Loth, F. L. C., Aaronson, N. K., et al. (2020). Thresholds for clinical importance were established to improve interpretation of the EORTC QLQ-C30 in clinical practice and research. The Journal of Clinical Epidemiology, 118, 1–8. https://doi.org/10.1016/j.jclinepi.2019.10.003.
Acknowledgements
The authors thank Dr Fiona Ecarnot who assisted in the proof-reading of the manuscript and English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
G.M. received honoraria from Novartis, Pfizer, Roche, BMS, and Ipsen. A.A. received travel fees from Novartis and Roche. The other authors have stated that they have no conflicts of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee (Est-II French Committee for Protection of Persons on January 25 2016 and the French national drug Agency (ANSM) on February 18 2016) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mouillet, G., Falcoz, A., Fritzsch, J. et al. Feasibility of health-related quality of life (HRQoL) assessment for cancer patients using electronic patient-reported outcome (ePRO) in daily clinical practice. Qual Life Res 30, 3255–3266 (2021). https://doi.org/10.1007/s11136-020-02721-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-020-02721-0