Abstract
Purpose
Patients with non-functioning pituitary adenomas (NFPA) suffer from pronounced impairments in physical and mental measures that result in an impairment of health-related quality of life (HRQOL). The role of secondary adrenal insufficiency (SAI) and especially the one of the hydrocortisone (HC) replacement dose on the HRQOL seems to be conflicting. The primary aim of this study is to assess the HRQOL in patients with NFPA in terms of presence of SAI and in patients without SAI and the secondary to explore the impact of treatment parameters such as daily HC dose.
Design/Methods
In a cross-sectional study we evaluated parameters of HRQOL in 95 patients with NFPA of the Endocrine Outpatient Unit of the Max Planck Institute of Psychiatry in Munich using standardized questionnaires like Short Form (SF-36), Beck's Depression Inventory (BDI), State-Trait Anxiety Inventory (STAI), Epworth Sleepiness Scale (ESS), Pittsburgh Sleep Quality Index (PSQI) and a self-constructed questionnaire about medical history.
Results
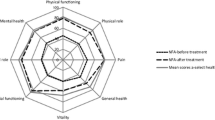
We could not find any significant difference between patients with and without SAI in the standardized questionnaires in terms of HRQOL. We could show that higher doses of HC were negatively correlated with HRQOL measured by SF-36 global health score regardless of using BDI or STAI in the block (β = − 0.397; p = 0.021, β = − 0.390; p = 0.016, respectively).
Conclusions
NFPA patients with SAI do not have a worse HRQOL than patients with NFPA and intact corticotropic axis. We could show that higher doses of HC are associated with an impaired HRQOL measured by SF-36 global and physical health score, whereas mental health score is not significantly influenced by the HC dose.
Similar content being viewed by others
References
Andela, C. D., Scharloo, M., Pereira, A. M., Kaptein, A. A., & Biermasz, N. R. (2015). Quality of life (QoL) impairments in patients with a pituitary adenoma: A systematic review of QoL studies. Pituitary, 18, 752–776.
Leistner, S. M., Klotsche, J., Dimopoulou, C., et al. (2015). Reduced sleep quality and depression associate with decreased quality of life in patients with pituitary adenomas. European Journal of Endocrinology, 172, 733–743.
van der Klaauw, A. A., Kars, M., Biermasz, N. R., et al. (2008). Disease-specific impairments in quality of life during long-term follow-up of patients with different pituitary adenomas. Clinical Endocrinology Oxford, 69, 775–784.
Ragnarsson, O., Mattsson, A. F., Monson, J. P., et al. (2014). The relationship between glucocorticoid replacement and quality of life in 2737 hypopituitary patients. European Journal of Endocrinology, 171, 571–579.
Milian, M., Honegger, J., Gerlach, C., & Psaras, T. (2013). Health-related quality of life and psychiatric symptoms improve effectively within a short time in patients surgically treated for pituitary tumors—A longitudinal study of 106 patients. Acta Neurochirurgica, 155, 1637–1645.
Tiemensma, J., Andela, C. D., Kaptein, A. A., et al. (2014). Psychological morbidity and impaired quality of life in patients with stable treatment for primary adrenal insufficiency: CROSS-sectional study and review of the literature. European Journal of Endocrinology, 171, 171–182.
Hahner, S., Loeffler, M., Fassnacht, M., et al. (2007). Impaired subjective health status in 256 patients with adrenal insufficiency on standard therapy based on cross-sectional analysis. The Journal of Clinical Endocrinology and Metabolism, 92, 3912–3922.
Danilowicz, K., Bruno, O. D., Manavela, M., Gomez, R. M., & Barkan, A. (2008). Correction of cortisol overreplacement ameliorates morbidities in patients with hypopituitarism: A pilot study. Pituitary, 11, 279–285.
Wichers, M., Springer, W., Bidlingmaier, F., & Klingmuller, D. (1999). The influence of hydrocortisone substitution on the quality of life and parameters of bone metabolism in patients with secondary hypocortisolism. Clinical Endocrinology Oxford, 50, 759–765.
Karppinen, A., Ritvonen, E., Roine, R., et al. (2016). Health-related quality of life in patients treated for non-functioning pituitary adenomas during the years 2000–2010. Clinical Endocrinology Oxford, 84(4), 532–539.
Romijn, J., Smit, J., & Lamberts, S. (2003). Intrinsic imperfections of endocrine replacement therapy. European Journal of Endocrinology, 149, 91–97.
Hahner, S., Loeffler, M., Bleicken, B., et al. (2010). Epidemiology of adrenal crisis in chronic adrenal insufficiency: The need for new prevention strategies. European Journal of Endocrinology, 162, 597–602.
Peacey, S. R., Guo, C. Y., Robinson, A. M., et al. (1997). Glucocorticoid replacement therapy: Are patients over treated and does it matter? Clinical Endocrinology Oxford, 46, 255–261.
Løvås, K., Gjesdal, C. G., Christensen, M., et al. (2009). Glucocorticoid replacement therapy and pharmacogenetics in Addison's disease: Effects on bone. European Journal of Endocrinology, 160, 993–1002.
Filipsson, H., Monson, J. P., Koltowska-Haggstrom, M., Mattsson, A., & Johannsson, G. (2006). The impact of glucocorticoid replacement regimens on metabolic outcome and comorbidity in hypopituitary patients. The Journal of Clinical Endocrinology and Metabolism, 91, 3954–3961.
Esteban, N. V., & Yergey, A. L. (1990). Cortisol production rates measured by liquid chromatography/mass spectrometry. Steroids, 55, 152–158.
Linder, B. L., Esteban, N. V., Yergey, A. L., Winterer, J. C., Loriaux, D. L., & Cassorla, F. (1990). Cortisol production rate in childhood and adolescence. The Journal of Pediatrics, 117, 892–896.
Ware, J. E., Jr., & Sherbourne, C. D. (1992). The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care, 30, 473–483.
Kühner, C., Bürger, C., Keller, F., & Hautzinger, M. (2007). Reliability and validity of the revised beck depression inventory (BDI-II). Der Nervenarzt, 78, 651–656.
Wang, Y.-P., & Gorenstein, C. (2013). Psychometric properties of the beck depression inventory-II: A comprehensive review. Revista Brasileira de Psiquiatria, 35, 416–431.
Bieling, P. J., Antony, M. M., & Swinson, R. P. (1998). The state-trait anxiety inventory, trait version: Structure and content re-examined. Behaviour Research and Therapy, 36, 777–788.
Caci, H., Baylé, F. J., Dossios, C., Robert, P., & Boyer, P. (2003). The Spielberger trait anxiety inventory measures more than anxiety. European Psychiatry, 18, 394–400.
Buysse, D. J., Reynolds, C. F., 3rd, Monk, T. H., Berman, S. R., & Kupfer, D. J. (1989). The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research, 28, 193–213.
Johns, M. W. (1991). A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep, 14, 540–545.
Johns, M. (1998). Rethinking the assessment of sleepiness. Sleep Medicine Reviews, 2, 3–15.
Ritvonen, E., Karppinen, A., Sintonen, H., et al. (2015). Normal long-term health-related quality of life can be achieved in patients with functional pituitary adenomas having surgery as primary treatment. Clinical Endocrinology Oxford, 82, 412–421.
Kluger, N., Matikainen, N., Sintonen, H., Ranki, A., Roine, R. P., & Schalin-Jantti, C. (2014). Impaired health-related quality of life in Addison's disease—impact of replacement therapy, comorbidities and socio-economic factors. Clinical Endocrinology Oxford, 81, 511–518.
Bleicken, B., Hahner, S., Loeffler, M., et al. (2010). Influence of hydrocortisone dosage scheme on health-related quality of life in patients with adrenal insufficiency. Clinical Endocrinology, 72, 297–304.
Morgan, S. A., Hassan-Smith, Z. K., & Lavery, G. G. (2016). Mechanisms in endocrinology: Tissue-specific activation of cortisol in Cushing's syndrome. European Journal of Endocrinology, 175(2), R83–R89.
Isidori, A. M., Venneri, M. A., Graziadio, C., et al. (2018). Effect of once-daily, modified-release hydrocortisone versus standard glucocorticoid therapy on metabolism and innate immunity in patients with adrenal insufficiency (DREAM): A single-blind, randomised controlled trial. Lancet Diabetes Endocrinology, 6(3), 173–185.
Funding
This study received no funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
CMW and MKA declare no conflict of interest. MS received speakers’ fees by Novartis, Ipsen, Pfizer, consultancy fees by Novartis and research funding by Novartis, Pfizer. APAK and GKS received consultancy fees and/or reimbursements of delegate fees for conferences/educational events and/or travel expenses and/or funding for research projects from Pfizer, Ipsen, Lilly, Shire, Novartis, Sandoz, NovoNordisk, and HRA.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Research involving human participants and/or animals
The study was an observational, non-interventional study involving human participants.
Informed consent
The study was approved by the medical ethics committee of the Ludwig-Maximilians-University of Munich. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wild, C.M., Stieg, M., Stalla, G.K. et al. Health-related quality of life in patients with non-functioning pituitary adenoma: a special focus on hydrocortisone replacement dose. Qual Life Res 29, 3325–3331 (2020). https://doi.org/10.1007/s11136-020-02582-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-020-02582-7