Abstract
Purpose
To assess improvements in health-related quality of life (HRQoL) with ixekizumab treatment in patients with moderate-to-severe psoriasis.
Methods
Adults with plaque psoriasis were enrolled in phase III, double-blind, randomised, controlled trials (UNCOVER-1, UNCOVER-2, or UNCOVER-3). All 3 protocols included a 12-week, placebo-controlled induction period; UNCOVER-2 and UNCOVER-3 also had an active-control group (50 mg etanercept) during induction. After induction, patients in UNCOVER-1 and UNCOVER-2 entered a 48-week withdrawal (maintenance) period (Weeks 12–60), during which Week-12 sPGA (0,1) responders were rerandomized to receive placebo, or 80 mg ixekizumab every 4 weeks (Q4W) or 12 weeks. As a secondary objective, HRQoL was measured by the generic Medical Outcomes Survey Short Form-36 (SF-36) at baseline and Weeks 12 and 60. Changes in mean SF-36 Physical and Mental Component Summary (PCS and MCS) and domain scores and proportions of patients reporting improvements ≥ minimal important differences in SF-36 scores were compared between groups.
Results
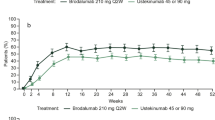
At Week 12, ixekizumab-treated patients (both dose groups in UNCOVER-1, -2, and -3) reported statistically significantly greater improvements in mean SF-36 PCS and MCS and all 8 SF-36 domain scores versus placebo. Further, more ixekizumab-treated patients than placebo-treated patients reported at least minimal treatment responses in SF-36 PCS and MCS scores and domain scores. Overall improvements in SF-36 PCS and MCS scores were maintained through Week 60.
Conclusions
Ixekizumab-treated patients reported statistically significant improvements in HRQoL at 12 weeks that persisted through 1 year.


Similar content being viewed by others
References
De Arruda, L. H. F., & De Moraes, A. P. F. (2001). The impact of psoriasis on quality of life. British Journal of Dermatology,144(Suppl 58), 33–36.
Fried, R. G., Friedman, S., Paradis, C., Hatch, M., Lynfield, Y., Duncanson, C., et al. (1995). Trivial or terrible? The psychosocial impact of psoriasis. International Journal of Dermatology,34(2), 101–105.
Krueger, G., Koo, J., Lebwohl, M., Menter, A., Stern, R. S., & Rolstad, T. (2001). The impact of psoriasis on quality of life: Results of a 1998 National Psoriasis Foundation patient-membership survey. Archives of Dermatology,137(3), 280–284.
Nash, A. S., McAteer, H., Schofield, J., Penzer, P., & Gilbert, A. K. (2015). Psoriasis today: Experiences of healthcare and impact on quality of life in a major UK cohort. Primary Health Care Research & Development,16(4), 415–423.
Kimball, A. B., Jacobson, C., Weiss, S., Vreeland, M. G., & Wu, Y. (2005). The psychosocial burden of psoriasis. American Journal of Clinical Dermatology,6(6), 383–392.
Molina-Leyva, A., Jiménez-Moleón, J. J., Naranjo-Sintes, R., & Ruiz-Carrascosa, J. C. (2015). Sexual dysfunction in psoriasis: A systematic review. Journal of the European Academy of Dermatology and Venereology,29(4), 649–655.
Dalgard, F. J., Gieler, U., Tomas-Aragones, L., Lien, L., Poot, F., Jemec, G. B., et al. (2015). The psychological burden of skin diseases: A cross-sectional multicenter study among dermatological out-patients in 13 European countries. Journal of Investigative Dermatology,135(4), 984–991.
Kurd, S. K., Troxel, A. B., Crits-Christoph, P., & Gelfand, J. M. (2010). The risk of depression, anxiety, and suicidality in patients with psoriasis: A population-based cohort study. Archives of Dermatology,146(8), 891–895.
Sampogna, F., Tabolli, S., Abeni, D., IDI Multipurpose Psoriasis Research on Vital Experiences (IMPROVE) investigators, et al. (2012). Living with psoriasis: prevalence of shame, anger, worry, and problems in daily activities and social life. Acta Dermato-Venereologica,92(3), 299–303.
Rapp, S. R., Feldman, S. R., Exum, M. L., Fleischer, A. B., Jr., & Reboussin, D. M. (1999). Psoriasis causes as much disability as other major medical diseases. Journal of the American Academy of Dermatology,41(3 Pt 1), 401–407.
Warren, R. B., Kleyn, C. E., & Gulliver, W. P. (2011). Cumulative life course impairment in psoriasis: Patient perception of disease-related impairment throughout the life course. British Journal of Dermatology,164(Suppl 1), 1–14.
Feldman, S. R., Gordon, K. B., Bala, M., Evans, R., Li, S., Dooley, L. T., et al. (2005). Infliximab treatment results in significant improvement in the quality of life of patients with severe psoriasis: A double-blind placebo-controlled trial. British Journal of Dermatology,152(5), 954–960.
Kimball, A. B., Bensimon, A. G., Guerin, A., Yu, A. P., Wu, E. Q., Okun, M. M., et al. (2011). Efficacy and safety of adalimumab among patients with moderate to severe psoriasis with co-morbidities: Subanalysis of results from a randomized, double-blind, placebo-controlled, phase III trial. American Journal of Clinical Dermatology,12(1), 51–62.
Krueger, G. G., Langley, R. G., Finlay, A. Y., Griffiths, C. E., Woolley, J. M., Lalla, D., et al. (2005). Patient-reported outcomes of psoriasis improvement with etanercept therapy: Results of a randomized phase III trial. British Journal of Dermatology,153(6), 1192–1199.
Langley, R. G., Elewski, B. E., Lebwohl, M., Reich, K., Griffiths, C. E., Papp, K., et al. (2014). Secukinumab in plaque psoriasis–results of two phase 3 trials. New England Journal of Medicine,371(4), 326–338.
Langley, R. G., Feldman, S. R., Han, C., Schenkel, B., Szapary, P., Hsu, M. C., et al. (2010). Ustekinumab significantly improves symptoms of anxiety, depression, and skin-related quality of life in patients with moderate-to-severe psoriasis: Results from a randomized, double-blind, placebo-controlled phase III trial. Journal of the American Academy of Dermatology,63(3), 457–465.
Lebwohl, M., Papp, K., Han, C., Schenkel, B., Yeilding, N., Wang, Y., et al. (2010). Ustekinumab improves health-related quality of life in patients with moderate-to-severe psoriasis: results from the PHOENIX 1 trial. British Journal of Dermatology,162(1), 137–146.
Revicki, D. A., Willian, M. K., Menter, A., Gordon, K. B., Kimball, A. B., Leonardi, C. L., et al. (2007). Impact of adalimumab treatment on patient-reported outcomes: Results from a Phase III clinical trial in patients with moderate to severe plaque psoriasis. Journal of Dermatological Treatment,18(6), 341–350.
Strand, V., Sharp, V., Koenig, A. S., Park, G., Shi, Y., Wang, B., et al. (2012). Comparison of health-related quality of life in rheumatoid arthritis, psoriatic arthritis and psoriasis and effects of etanercept treatment. Annals of the Rheumatic Diseases,71(7), 1143–1150.
Feldman, S. R., & Krueger, G. G. (2005). Psoriasis assessment tools in clinical trials. Annals of the Rheumatic Diseases,64(Suppl 2), ii65–ii68. (discussion ii69–73).
Mrowietz, U., Kragballe, K., Reich, K., Spuls, P., Griffiths, C. E., Nast, A., et al. (2011). Definition of treatment goals for moderate to severe psoriasis: A European consensus. Archives of Dermatological Research,303(1), 1–10.
Ware, J. E., Jr., & Sherbourne, C. D. (1992). The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care,30(6), 473–483.
Di Cesare, A., Di Meglio, P., & Nestle, F. O. (2009). The IL-23/Th17 axis in the immunopathogenesis of psoriasis. Journal of Investigative Dermatology,129(6), 1339–1350.
Genovese, M. C., Van den Bosch, F., Roberson, S. A., Bojin, S., Biagini, I. M., Ryan, P., et al. (2010). LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheumatology,62(4), 929–939.
Leonardi, C., Matheson, R., Zachariae, C., Cameron, G., Li, L., & Edson-Heredia, E. (2012). Anti-interleukin-17 monoclonal antibody ixekizumab in chronic Plaque psoriasis. New England Journal of Medicine,366(13), 1190–1199.
Liu, L., Lu, J., Allan, B. W., Tang, Y., Tetreault, J., Chow, C. K., et al. (2016). Generation and characterization of ixekizumab, a humanized monoclonal antibody that neutralizes interleukin-17A. Journal of Inflammation Research,9, 39–50.
Griffiths, C. E. M., Reich, K., Lebwohl, M., van der Kerkhof, P., Paul, C., Menter, A., et al. (2015). Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): Results from two phase 3 randomised trials. The Lancet,386(9993), 541–551.
Gordon, K. B., Blauvelt, A., Papp, K. A., Langley, R. G., Luger, T., Ohtsuki, M., et al. (2016). Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. New England Journal of Medicine,375(4), 345–356.
Maruish, M. E. (Ed.). (2011). User’s manual for the SF36v2 Health Survey (3rd ed.). Lincoln, RI: Quality Metric Incorporated.
Ixekizumab (TALTZ™) Package Insert. Eli Lilly and Company. Indianapolis, IN USA. Revised January 2017.
Prakash, A., Risser, R. C., & Mallinckrodt, C. H. (2008). The impact of analytic method on interpretation of outcomes in longitudinal clinical trials. International Journal of Clinical Practice,62(8), 1147–1158.
Acknowledgements
This study was sponsored by Eli Lilly and Company. We are indebted to the patients and study personnel who participated in UNCOVER-1, UNCOVER-2, and UNCOVER-3. Dr. Richard Warren is supported by the NIHR Manchester Biomedical Research Centre. We thank Lori Kornberg, PhD, Kelly Guerrettaz, and Lydia Morris, PhD, of Syneos Health, Raleigh, NC, who provided writing support on behalf of Eli Lilly and Company. We also thank Vrishali Lopes of Optum Outcomes Insight Consulting, Lincoln, RI, and Regina Rendas-Baum of Optum Quality Metrics, Lincoln, RI, for their data analysis on SF-36 population norms for the USA on behalf of Eli Lilly and Company.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ms Nikaï and Edson-Heredia, and Drs Zhu, Goldblum, Carlier, Burge, Lin, and Hollister are employees of Eli Lilly and Company and own stock. Professor Langley is a consultant for AbbVie, Amgen, Celgene, Pfizer, Janssen, Boehringer Ingleheim, LEO Pharma, and Valeant. He has received grants/has pending grants, has received honoraria, has received payment for development of educational programs including speaker bureau service, and received expenses covered or reimbursed for travel from AbbVie, Amgen, Celgene, Pfizer, Janssen, Boehringer Ingleheim, and LEO Pharma. Professor Reich has served as advisor and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Affibody, Almirall, Amgen, Avillion, Biogen, Boehringer Ingelheim Pharma, Celgene, Centocor, Covagen, Forward Pharma, Fresenius Medical Care, Galapagos, GlaxoSmithKline, Janssen-Cilag, Kyowa Kirin, LEO Pharma, Eli Lilly and Company, Medac, Merck Sharp & Dohme Corp., Novartis, Miltenyi Biotec, Ocean Pharma, Pfizer, Regeneron, Samsung Bioepis, Sanofi, Sun Pharma, Takeda, UCB, Valeant, and XenoPort. Dr Feldman reports that a third party that was supported by Eli Lilly and Company assisted with manuscript preparation. He is a consultant for Eli Lilly and Company, Novartis, Janssen, and Celgene. He received Honoria from Eli Lilly and Company, AbbVie, Celgene, Janssen, and Novartis. He reports grant support or pending grant support from Eli Lilly and Company, Novartis, Janssen, AbbVie, and Celgene (support paid to institution). Dr. Strand reports consulting fees from Eli Lilly and Company, AbbVie, Amgen, Anthera, AstraZeneca, BMS, Boehringer Ingelheim, Celltrion, EMD Serono, Genentech/Roche, GSK, Janssen, Novartis, Pfizer, Regeneron, Sanofi, UCB, outside the submitted work. Dr Paul has received grant support from ADERMI. He reports honoria and reimbursed travel expenses from AbbVie, Boerhinger, Celgene, Eli Lilly and Company, Janssen Cilag, Almirall, Regeneron, Sanofi, Novartis, Pfizer, LEO Pharma, and Pierre Fabre. Dr Gordon received research support from AbbVie, Boehringer Ingelheim, Celgene, Eli Lilly and Company, Novartis, and Jannsen. He has received consulting fees or honoraria from AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, Novartis, Janssen, and Eli Lilly and Company. Dr Richard Warren has actively consulted for AbbVie, Almiral, Amgen, Boehringer Ingelheim, Celgene, Pfizer, Novartis, Janssen, LEO Pharma, XenPort, Eli Lilly and Company, and UCB. Dr Warren’s institution has grants/grants pending from LEO Pharma, AbbVie, Novartis, and Eli Lilly and Company. Dr Warren has received an honorarium from AbbVie, Almiral, Amgen, Boehringer Ingelheim, Celgene, Pfizer, Novartis, Janssen, LEO Pharma, XenPort, Eli Lilly and Company, and UCB. Dr Toth has served as an investigator or speaker for Abbvie, Amgen, BMS, Boehringer Ingelheim, Celgene, Elli Lilly and Company, Incyte, Janssen, Leo Pharma, Novartis, Pfizer, Regeneron, Sanofi-Genzyme, and UCB. M. Augustin has served as a consultant and/or paid speaker for and/or has received research grants and/or honoraries for consulting and/or scientific lectures for and/or received travel expense reimbursement and/or participated in clinical trials sponsored by companies that manufacture drugs used for treatment of psoriasis including AbbVie, Almirall, Amgen, Biogen (Biogen Idec), Boehringer Ingelheim, Celgene, Centocor, Eli Lilly and Company, Janssen-Cilag, LEO Pharma, Medac, MSD (formerly Essex, Schering-Plough), Mundipharma, Novartis, Pfizer, (formerly Wyeth), Pohl Boskamp, Sandoz, and XenoPort.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committees and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Langley, R.G.B., Reich, K., Strand, V. et al. Ixekizumab treatment and the impact on SF-36: results from three pivotal phase III randomised controlled trials in patients with moderate-to-severe plaque psoriasis. Qual Life Res 29, 369–380 (2020). https://doi.org/10.1007/s11136-019-02296-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-019-02296-5