Abstract
Purpose
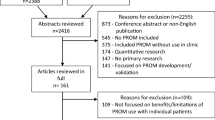
The use of patient-reported outcome (PRO) measures in clinical practice is increasing. Following the creation of a ‘User’s Guide to Implementing PRO Assessment in Clinical Practice’ by the International Society for Quality of Life Research (ISOQOL), volunteers from ISOQOL sought to create a Companion Guide to assist health care providers with the scientific and practical considerations involved in implementing and using PRO measures in clinical care by using information from real-world case studies. This paper summarizes the key issues presented in the Companion Guide.
Methods
Ten respondents, who were members of the ISOQOL’s CP-SIG and worked in various clinical areas, participated in a survey or telephone interview. Participants were from Canada (n = 2), Denmark (n = 1), England (n = 2), Holland (n = 1), and the United States (n = 4).
Results
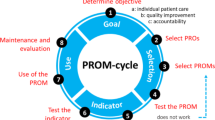
Based on the information provided by respondents, a Companion Guide was produced, organized according to the nine questions presented in the User’s Guide. An additional section for key take-home messages was also provided. This guide provides examples of issues and considerations related to the implementation of PRO measures in clinical practice.
Conclusions
Respondents provided insight into their experiences and emphasized that PRO initiatives were likely to be more successful if there is purposeful, designed integration into clinical practice, meaningful substantive engagement with all stakeholders and access to necessary organizational resources. The ability to leverage existing technology as well as realistic and stakeholder consensus-driven expectations for planning and timing were also key to the successful implementation of PRO measures.
Similar content being viewed by others
References
Chen, J., Ou, L., & Hollis, S. J. (2013). A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Services Research, 13, 211.
Velikova, G., Booth, L., Smith, A. B., Brown, P. M., Lynch, P., Brown, J. M., et al. (2004). Measuring quality of life in routine oncology practice improves communication and patient well-being: A randomized controlled trial. Journal of Clinical Oncology, 22(4), 714–724.
Boyce, M. B., Browne, J. P., & Greenhalgh, J. (2014). The experiences of professionals with using information from patient-reported outcome measures to improve the quality of healthcare: A systematic review of qualitative research. BMJ Quality and Safety, 23(6), 508–518.
Haywood, K. L., Garratt, A. M., Carrivick, S., Magnall, J., & Skevington, S. M. (2009). Continence specialists use of quality of life information in routine practice: A national survey of practitioners. Quality of Life Research, 18(4), 423–433.
Lohr, K. N., & Zebrack, B. J. (2009). Using patient-reported outcomes in clinical practice: Challenges and opportunities. Quality of Life Research, 18(1), 99–107.
Valderas, J. M., Kotzeva, A., Espallargues, M., Guyatt, G., Ferrans, C. E., Halyard, M. Y., et al. (2008). The impact of measuring patient-reported outcomes in clinical practice: A systematic review of the literature. Quality of Life Research, 17, 179–193.
International Society for Quality of Life Research. (2015). User’s guide to implementing patient-reported outcomes assessment in clinical practice, version 2. Retrieved March 27, 2018, from http://www.isoqol.org/UserFiles/2015UsersGuide-Version2.pdf.
Guest, G., MacQueen, K. M., & Namey, E. E. (2011). Applied thematic analysis. Thousand Oaks, CA: Sage.
U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. (2006). Guidance for industry: patient-reported outcome measures: Use in medical product development to support labeling claims: Draft guidance. Health Quality of Life Outcomes, 4(1), 79.
Committee for Medicinal Products for Human Use. (2005). Reflection paper on the regulatory guidance for the use of health-related quality of life (HRQL) measures in the evaluation of medicinal products. London: European Medicines Agency.
World Health Organization WH. (2001). International classification of functioning. Disability: ICF. World Health Organization.
Wilson, I. B., & Cleary, P. D. (1995). Linking clinical variables with health-related quality of life: A conceptual model of patient outcomes. Jama, 273(1), 59–65.
Berwick, D. M., Nolan, T. W., & Whittington, J. (2008). The triple aim: Care, health, and cost. Health Affairs, 27(3), 759–769.
Acknowledgements
This paper is produced on behalf of the International Society for Quality of Life Research (ISOQOL). All authors are members of ISOQOL. The manuscript was reviewed and approved by the ISOQOL Board of Directors as an ISOQOL publication and does not reflect an endorsement of the ISOQOL membership. We would like to acknowledge the time and effort of our case study contributors in supporting the creation of this Companion Guide.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
It was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Chan, E.K.H., Edwards, T.C., Haywood, K. et al. Implementing patient-reported outcome measures in clinical practice: a companion guide to the ISOQOL user’s guide. Qual Life Res 28, 621–627 (2019). https://doi.org/10.1007/s11136-018-2048-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-018-2048-4