Abstract
Purpose
The cancer anorexia–cachexia syndrome (CACS) is highly prevalent in lung cancer (LC) patients (57–61%), and represents the direct cause of death in 20% of cases. Accurately quantifying CACS has been a challenging issue; consequently, this study presents the clinical validation of the Spanish version of the Functional Assessment of Anorexia–Cachexia Therapy (FAACT) scale in LC patients from Latin America.
Methods
The Spanish version of the FAACT and the Mexican-Spanish version of the EORTC-QLQ-C30 instruments were applied to a cohort of patients with LC at the National Cancer Institute of Mexico. Reliability and validity tests were performed to assess the psychometric properties of the scales, and clinical validation was assessed considering the association of scales with subjective and objective clinical data.
Results
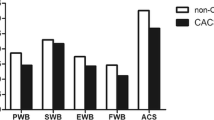
Two hundred patients were included. Questionnaire compliance rates were high (100%) and the instrument was well accepted in all cases; internal consistency tests demonstrated good convergent and divergent validity of the scale structure. Cronbach’s α coefficient for three out of five basic multi-item scales was > 0.7 (0.55–0.86). FAACT scales presented significant associations with clinical parameters, including biochemical and nutritional variables (i.e., energy intake, p = 0.002), as well as strongly correlated with the appetite loss subscale of the QLQ-C30 questionnaire (r = − 0.624). Physical well-being (p < 0.0009), functional well-being (p = 0.004), anorexia/cachexia scale (p = 0.029), and FAACT total scores (p = 0.0009) were strongly associated to overall survival.
Conclusion
The Spanish version of the FAACT questionnaire is reliable and valid for the assessment of health-related quality of life and CACS in LC patients and can be used in clinical trials.


Similar content being viewed by others
References
Torre, L. A., Bray, F., Siegel, R. L., Ferlay, J., Lortet-Tieulent, J., & Jemal, A. (2015). Global cancer statistics, 2012. Cancer Journal For Clinicians, 65(2), 87–108. https://doi.org/10.3322/caac.21262.
Salsman, J. M., Beaumont, J. L., Wortman, K., Yan, Y., Friend, J., & Cella, D. (2015). Brief versions of the FACIT-fatigue and FAACT subscales for patients with non-small cell lung cancer cachexia. Supportive Care in Cancer, 23(5), 1355–1364. https://doi.org/10.1007/s00520-014-2484-9.
Sanchez-Lara, K., Turcott, J. G., Juarez-Hernandez, E., Nunez-Valencia, C., Villanueva, G., Guevara, P., et al. (2014). Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: Randomised trial. Clinical Nutrition, 33(6), 1017–1023. https://doi.org/10.1016/j.clnu.2014.03.006.
Arrieta, O., Michel Ortega, R. M., Villanueva-Rodriguez, G., Serna-Thome, M. G., Flores-Estrada, D., Diaz-Romero, C., et al. (2010). Association of nutritional status and serum albumin levels with development of toxicity in patients with advanced non-small cell lung cancer treated with paclitaxel-cisplatin chemotherapy: A prospective study. BMC Cancer, 10, 50. https://doi.org/10.1186/1471-2407-10-50.
Sanchez-Lara, P. A., Zhao, H., Bajpai, R., Abdelhamid, A. I., & Warburton, D. (2012). Impact of stem cells in craniofacial regenerative medicine. Frontiers in Physiology, 3, 188. https://doi.org/10.3389/fphys.2012.00188.
Kovarik, M., Hronek, M., & Zadak, Z. (2014). Clinically relevant determinants of body composition, function and nutritional status as mortality predictors in lung cancer patients. Lung Cancer, 84(1), 1–6. https://doi.org/10.1016/j.lungcan.2014.01.020.
Fearon, K., Strasser, F., Anker, S. D., Bosaeus, I., Bruera, E., Fainsinger, R. L., et al. (2011). Definition and classification of cancer cachexia: An international consensus. The Lancet Oncology, 12(5), 489–495. https://doi.org/10.1016/S1470-2045(10)70218-7.
Blum, D., Stene, G. B., Solheim, T. S., Fayers, P., Hjermstad, M. J., Baracos, V. E., et al. (2014). Validation of the consensus-definition for cancer cachexia and evaluation of a classification model: A study based on data from an international multicentre project (EPCRC-CSA). Annals of Oncology, 25(8), 1635–1642. https://doi.org/10.1093/annonc/mdu086.
Del Ferraro, C., Grant, M., Koczywas, M., & Dorr-Uyemura, L. A. (2012) Management of anorexia–cachexia in late stage lung cancer patients. Journal of Hospice and Palliative Nursing. https://doi.org/10.1097/NJH.0b013e31825f3470.
Teunissen, S. C., Wesker, W., Kruitwagen, C., de Haes, H. C., Voest, E. E., & de Graeff, A. (2007). Symptom prevalence in patients with incurable cancer: A systematic review. Journal of Pain and Symptom management, 34(1), 94–104. https://doi.org/10.1016/j.jpainsymman.2006.10.015.
Argiles, J. M. (2005) Cancer-associated malnutrition. European Journal of Oncology Nursing, 9(Suppl 2), S39–S50. https://doi.org/10.1016/j.ejon.2005.09.006.
Tisdale, M. J. (2002). Cachexia in cancer patients. Nature Reviews Cancer, 2(11), 862–871. https://doi.org/10.1038/nrc927.
Skipworth, R. J., Stewart, G. D., Dejong, C. H., Preston, T., & Fearon, K. C. (2007). Pathophysiology of cancer cachexia: Much more than host–tumour interaction? Clinical Nutrition, 26(6), 667–676. https://doi.org/10.1016/j.clnu.2007.03.011.
Pilkington, G., Boland, A., Brown, T., Oyee, J., Bagust, A., & Dickson, R. (2015). A systematic review of the clinical effectiveness of first-line chemotherapy for adult patients with locally advanced or metastatic non-small cell lung cancer. Thorax, 70(4), 359–367. https://doi.org/10.1136/thoraxjnl-2014-205914.
Therasse, P., Arbuck, S. G., Eisenhauer, E. A., Wanders, J., Kaplan, R. S., Rubinstein, L., et al. (2000). New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute, 92(3), 205–216.
Basch, E., Geoghegan, C., Coons, S. J., Gnanasakthy, A., Slagle, A. F., Papadopoulos, E. J., et al. (2015). Patient-reported outcomes in cancer drug development and US Regulatory Review: Perspectives from industry, the food and drug administration, and the patient. JAMA Oncology, 1(3), 375–379. https://doi.org/10.1001/jamaoncol.2015.0530.
Wheelwright, S., Darlington, A. S., Hopkinson, J. B., Fitzsimmons, D., White, A., & Johnson, C. D. (2013). A systematic review of health-related quality of life instruments in patients with cancer cachexia. Supportive Care in Cancer, 21(9), 2625–2636. https://doi.org/10.1007/s00520-013-1881-9.
LeBlanc, T. W., Samsa, G. P., Wolf, S. P., Locke, S. C., Cella, D. F., & Abernethy, A. P. (2015) Validation and real-world assessment of the Functional Assessment of Anorexia–Cachexia Therapy (FAACT) scale in patients with advanced non-small cell lung cancer and the cancer anorexia–cachexia syndrome (CACS). Supportive Care in Cancer 23(8), 2341–2347. https://doi.org/10.1007/s00520-015-2606-z.
Edge, S. B., & Compton, C. C. (2010). The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of Surgical Oncology, 17(6), 1471–1474. https://doi.org/10.1245/s10434-010-0985-4.
Chang, V. T., Xia, Q., & Kasimis, B. (2005). The Functional Assessment of Anorexia/Cachexia Therapy (FAACT) appetite scale in veteran cancer patients. The Journal of Supportive Oncology, 3(5), 377–382.
Ribaudo, J. M., Cella, D., Hahn, E. A., Lloyd, S. R., Tchekmedyian, N. S., Von Roenn, J., et al. (2000). Re-validation and shortening of the Functional Assessment of Anorexia/Cachexia Therapy (FAACT) questionnaire. Quality of life research. Care and Rehabilitation, 9(10), 1137–1146.
Hernandez-Avila, M., Romieu, I., Parra, S., Hernandez-Avila, J., Madrigal, H., & Willett, W. (1998). Validity and reproducibility of a food frequency questionnaire to assess dietary intake of women living in Mexico City. Salud publica de Mexico, 40(2), 133–140.
Muscaritoli, M., Anker, S. D., Argiles, J., Aversa, Z., Bauer, J. M., et al. (2010). Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clinical Nutrition, 29(2), 154–159. https://doi.org/10.1016/j.clnu.2009.12.004.
Arrieta, O., Nunez-Valencia, C., Reynoso-Erazo, L., Alvarado, S., Flores-Estrada, D., Angulo, L. P., et al. (2012). Health-related quality of life in patients with lung cancer: Validation of the Mexican-Spanish version and association with prognosis of the EORTC QLQ-LC13 questionnaire. Lung Cancer, 77(1), 205–211. https://doi.org/10.1016/j.lungcan.2012.02.005.
Fayers, P. M. M. D. (2000). Quality of life: Assessment, analysis and interpretation. Chichester: Wiley.
Bauer, J., Capra, S., & Ferguson, M. (2002). Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. European Journal of Clinical Nutrition, 56(8), 779–785. https://doi.org/10.1038/sj.ejcn.1601412.
Tabachnick, B. G., & Fidell, L. S. (2014). Using multivariate statistics (6th edn.). Jarlow: Pearson.
Sanchez-Lara, K., Turcott, J. G., Juarez, E., Guevara, P., Nunez-Valencia, C., Onate-Ocana, L. F., et al. (2012). Association of nutrition parameters including bioelectrical impedance and systemic inflammatory response with quality of life and prognosis in patients with advanced non-small-cell lung cancer: A prospective study. Nutrition and Cancer, 64(4), 526–534. https://doi.org/10.1080/01635581.2012.668744.
Acknowledgements
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Supportive Care in Cancer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Oscar Arrieta has received payment for lectures from Pfizer, AstraZeneca, Boehringer-Ingelheim, and Roche. Julissa Luvian-Morales declares no conflict of interest. Jenny G. Turcott declares no conflict of interest. Luis F. Oñate-Ocaña declares no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Arrieta, O., Luvián-Morales, J., Turcott, J.G. et al. Quality of life and anorexia/cachexia in lung cancer: validation of the Spanish version of the FAACT instrument. Qual Life Res 27, 2709–2718 (2018). https://doi.org/10.1007/s11136-018-1930-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-018-1930-4